OP35 Treatment outcomes of inflammatory bowel disease in the biological era—a nationwide retrospective cohort study in three Nordic countries: Results from the TRINordic study
M. Zhao1, M. Lördal2, E. Langholz3, T. Knudsen4, M. Voutilainen5, M.L. Høivik6, B. Moum6, B. Saebo7, P. Haiko8, C. Malmgren9, M. Coskun10, H.O. Melberg11, J. Burisch12
1Gastrointestinal Unit, Hvidovre Hospital- Copenhagen University, Hvidovre, Denmark, 2Department of Gastroenterology and Hepatology, Danderyds Hospital, Stockholm, Sweden, 3Department of Medical Gastroenterology, Herlev Hospital, Herlev, Denmark, 4Department of Medicine,- Hospital South West Denmark; Department of Regional Health Research- University of Southern Denmark, Odense, Denmark, 5Department of Gastroenterology, University of Turku, Turku, Finland, 6Department of Gastroenterology, Oslo University Hospital, Oslo, Norway, 7Takeda AS, Medical Affairs, Asker, Norway, 8Takeda Oy, Medical Affairs, Helsinki, Finland, 9Takeda Pharma AB, Medical Affairs, Stockholm, Sweden, 10Takeda A/S, Medical Affairs, Taastrup, Denmark, 11Department of Health Management and Health Economics, Institute of Health and Society, University of Oslo, Oslo, Norway, 12Gastrounit- Medical Division, Hvidovre University Hospital, Hvidovre, Denmark
Background
Biological therapy has been suggested to decrease surgery and hospitalisation risk in patients diagnosed with inflammatory bowel disease (IBD). During 2010 to 2016, the use of biologics in Denmark (DEN), Sweden (SWE) and Norway (NOR) increased dramatically and the time to first biologic treatment declined.1 However, the impact of increasing use of biologics on disease outcomes remains to be shown in real-life practice. In this nationwide study in three Nordic countries, we aimed to investigate trends in surgery and hospitalisation rates in IBD patients in the biological era.1 Høivik ML
Methods
A total number of 67 758 IBD patients (42 894 patients with ulcerative colitis (UC) and 24 864 Crohn’s disease (CD) diagnosed during the period from 2010 to 2017 in DEN, NOR and SWE were included using the National Patient Registries. Patients were required to have 1-year follow-up; results are limited to patients diagnosed between 2010 and 2016, inclusive. Using the unique personal identification number, individual-level information on surgery, hospitalisation and drug treatment were extracted from the National Patient Registries and the National Prescription Registries. Disease outcomes within two years after diagnosis were compared across annual cohorts.
Results
During 2010 to 2016, 2-year surgery rates in CD patients showed a non-significant decline from 11.9% in 2011 to 9.5% in 2016 in SWE while remaining stable in NOR and DEN (Figure 1). No temporal pattern in surgery risk was observed for UC. The proportion of CD patients being hospitalised within two years from diagnosis declined in SWE and NOR from 52.3% and 51.0% in 2011 to 47.3% and 38.5% in 2015 (
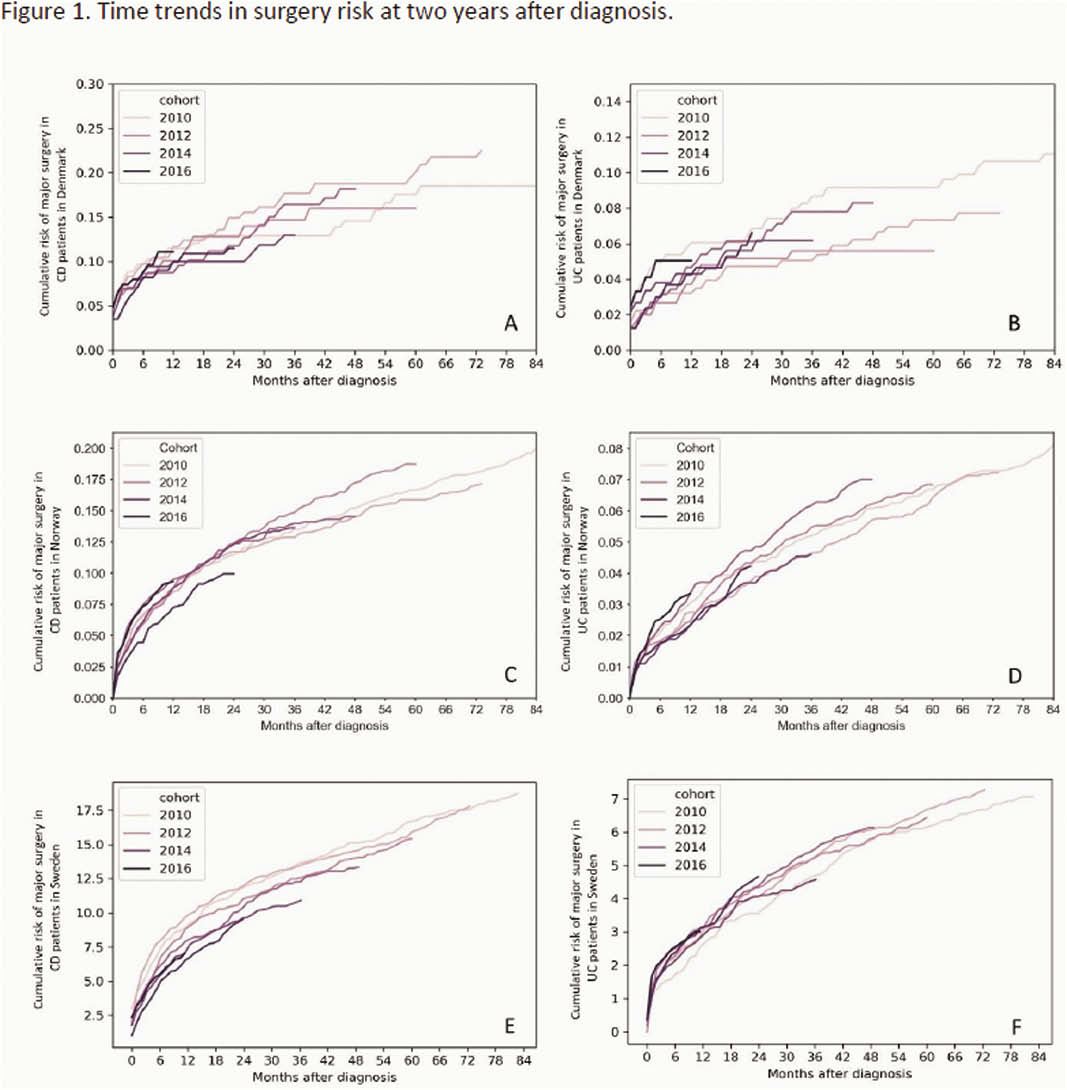
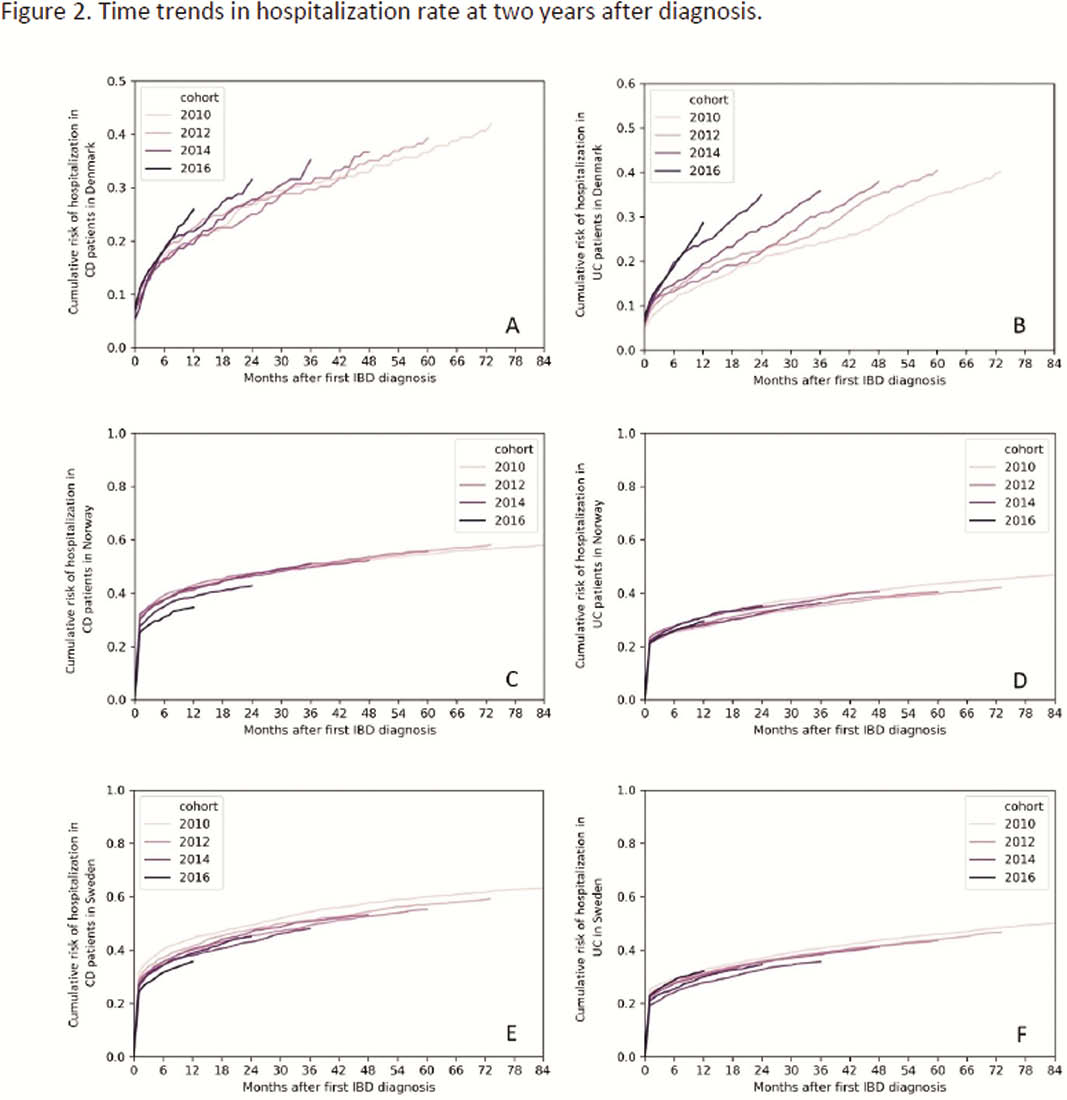
Conclusion
No clear pattern was seen in two-year surgery and hospitalisation rates in IBD patients during 2010 to 2017 despite a concurrent increase in biological use in all countries. However, differences in treatment practices across countries might influence these findings. The impact of increased biological use on long-term outcomes in IBD remains to be shown.


