Preclinical IBD: the key to the future is (probably) behind us
Iago Rodríguez-Lago, EpiCom Member
|
Iago Rodríguez-Lago |
Introduction
The incidence and prevalence of Inflammatory Bowel Disease (IBD) are progressively increasing worldwide, particularly in developing countries. Despite increasing awareness of IBD and improvements in biomarkers and diagnostic techniques, a significant diagnostic delay is still frequently observed. This is highly relevant as diagnostic delay prevents application of medical treatment during very early disease stages and the implementation of certain disease intervention strategies. Diagnostic delay is consequently still limiting our potential to alter the natural history of the disease, as has recently been shown by UK colleagues. In a systematic review and meta-analysis, it was demonstrated that individuals with Crohn’s Disease (CD) in the higher quartiles of diagnostic delay (median 24 months) were more likely to have stricturing or penetrating disease and were also more likely to undergo intestinal surgery, while in patients with Ulcerative Colitis (UC) such a delay was associated with increased probability of colectomy [1]. Hence, reducing diagnostic delay should be a priority if we are aiming to apply effective disease intervention strategies.
In the meantime, our understanding of the mechanisms underlying the development of IBD have improved significantly. It is now clear that IBD leads to cumulative damage in the bowel wall, and that this is associated with significant disability and decreased quality of life. However, those events occurring at the very onset of the pathogenic process have not yet been clearly elucidated. Improving our understanding of these early events would enable us to identify the main triggers of the inflammatory process and the drivers that lead to a chronic and persistent immunological response. More importantly, it would also reveal which targets will offer greater benefit when treating the disease early, and potentially could even make possible the application of preventive measures in subjects at risk.
In recent years, international collaborative initiatives have provided many significant clinical and basic data and this new evidence has been received with great interest. The Seventh Scientific Workshop of ECCO on Precision Medicine consequently addressed the topic of prediction and prevention of IBD [2]. The panel of international experts established definitions, reviewed the current evidence and proposed what should be considered by future work in this field (Figure 1). For instance, various studies have now demonstrated that events during the first years of life , such as smoking, infections and antibiotic use, can increase susceptibility to IBD development [3]. In addition, it has been demonstrated that certain immunological, clinical and healthcare consequences are already present in the years before diagnosis, even during the period without detectable disease in terms of gastrointestinal symptoms [4–6].
Figure 1. Overview of the stages during the preclinical period of IBD (adapted from Torres et al. [2])
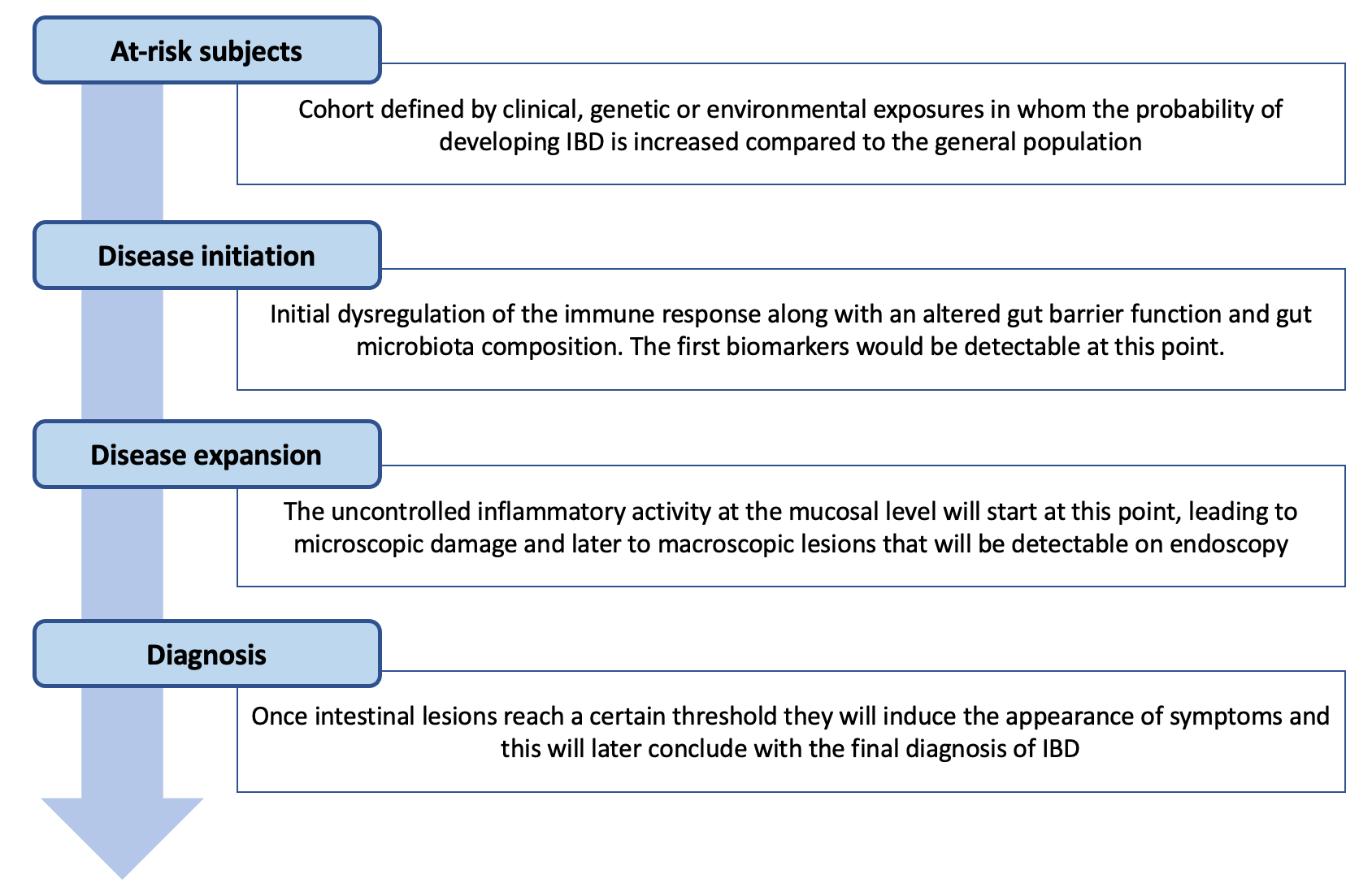
The concept of preclinical IBD and its staging
The most widely accepted definition of preclinical IBD includes all the events and findings occurring before the clinical onset of disease [2]. Many different studies involving analysis of serological and proteomic panels [4, 7–11], metabolomic features [12], microbiome signatures [13] and faecal markers [14] have built a robust body of evidence showing that is already present years before diagnosis, and these findings have been linked to certain altered cellular pathways [4]. Overall, the collective evidence nicely demonstrates how a systemic yet subclinical immune process can be present for a long time in asymptomatic subjects (disease initiation phase), confirming such unique opportunity for earlier disease identification.
The next phase (i.e. disease expansion) involves progression of the pathogenic process within the bowel wall and at the mucosal level. Identification of this pathogenic process might seem (very) complicated at first sight, but the introduction of regional and nationwide colorectal cancer screening programmes has shown that it is possible to detect mucosal damage associated with IBD in asymptomatic subjects after a positive faecal occult blood test [15–17]. Interestingly, this subclinical inflammation is also linked with an overall increase in the burden on healthcare resources, including primary care visits and steroid use [5]. This context provides an opportunity to analyse how an altered interaction between the host, environmental factors and gut microbiota evolves and leads to the initial lesions, even before a person describes the initial manifestations.
Several studies have evaluated the preclinical period and some are still ongoing, so more results can be expected in the near future [18, 19]. Here we highlight some of these studies:
PREDICTS (PRoteomic Evaluation and Discovery in an IBD Cohort of Tri-service Subjects)
This is a nested case-control study of subjects with incident IBD, including 1000 cases of CD, 1000 cases of UC and 500 healthy controls from the Defense Medical Surveillance System [20]. This is the main database from the US Armed Forces and contains clinical information from over 10 million members who have served in the armed forces since 1990. Up to four serum samples obtained biennially have been obtained from cases and controls.
This repository has led to the discovery of many of the most relevant findings in the field of preclinical IBD, including proteomic markers [4] and a range of serologic markers, such as antimicrobial antibodies [4] and anti-GM-CSF antibodies [9]. Most of the evidence relates to CD patients, and the immune process underlying UC remains partially undiscovered. However, recent data from this team of investigators have demonstrated that certain anti-integrin antibodies towards integrin αvβ6 are also present before the onset of UC [7]. Further investigations are ongoing from this cohort and we all are expecting more results from these researchers.
CCC GEM (Crohn’s and Colitis Canada Genetic, Environmental, Microbial) cohort
This study, led by Crohn’s and Colitis Canada, involves a prospective cohort including asymptomatic first-degree relatives of CD patients aged between 6 and 35 years. After excluding any digestive symptoms and previous gastrointestinal disease, these subjects have been followed up every six months (https://www.gemproject.ca/). Highly interesting findings have been obtained from a range of analyses comparing the characteristics of first-degree relatives who have developed CD and those who have remained asymptomatic (without IBD) during follow-up.
These researchers have demonstrated different aspects of the subclinical inflammatory process that develops prior to diagnosis in this selected at-risk population. In a cohort of 1420 first-degree relatives who were followed up for a median of 7.8 years, they nicely showed how disruption of gut barrier integrity can be detected through an increase in intestinal permeability years before diagnosis, and this link remained significant even if the test was performed more than three years before CD diagnosis [21]. Additionally, more recent data have shown that the altered gut barrier function is linked to a different gut microbiome and a proteomic signature [10, 22]. Interestingly, these researchers have also replicated the findings from the PREDICTS group showing that antimicrobial antibodies are present in these patients, so they could be included as additional biomarkers [23]. In view of these findings, we may conclude that the in-depth evaluation of first-degree relatives within the CCC GEM project will definitely increase our knowledge about the initial processes prior to disease onset.
POP-IBD
The POP-IBD study group is a collaborative enterprise involving researchers from St George's University of London, Imperial College London, University College London and King's College London. The group conducts population-based studies in the field of IBD, and among these it is worthwhile to highlight those concerning the presence of gastrointestinal symptoms and psychiatric comorbidity before IBD diagnosis. These researchers have described an excess prevalence of digestive symptoms over the ten years preceding IBD diagnosis [24]. Also, the association of these symptoms with depression increased the risk of both UC and CD, by 47% and 41%, respectively [25]. Their clinical perspective in approaching the preclinical period through population-based studies is of great value and will help in determining those individuals who are at higher risk of developing IBD and will need early referral to specialised clinics.
PREDICT (Center for Molecular Prediction of Inflammatory Bowel Disease)
The Center for the Molecular Prediction of Inflammatory Bowel Disease (PREDICT) is a national centre of excellence at Aalborg University, Copenhagen, Denmark. The purpose of PREDICT is to study and unravel the cause and prognosis of IBD. Based on unique patient samples from the Danish National Biobank combined with longitudinal nationwide register data, PREDICT examines genetics, epigenetics, antibodies, inflammatory markers, metabolomics and microbiomes in thousands of patients prior to development of IBD. PREDICT has built a data infrastructure with nationwide registry data and biological data from biobank samples. Data from biobank samples include GWAS (genome-wide association study) data on >9000 patients with IBD and inflammatory markers on neonatal blood spots from >400 individuals who later developed IBD; in addition, metabolomics studies are currently being conducted on 4000 prediagnostic IBD serum samples.
Recently PREDICT showed that patients with IBD have higher use of almost every medication ten years prior to IBD diagnosis, indicating multiorgan involvement of IBD [6]. Also, the centre has described increased use of antibiotics prior to development of IBD [26] and has found that patients with IBD are at increased risk of anxiety and depression both before and after the diagnosis of IBD [27]. The PREDICT data infrastructure represents a unique and well-powered framework which will be leveraged for further investigations of the prediagnostic phase of IBD.
EARLY cohort
The Spanish IBD Working Group on Crohn’s Disease and Ulcerative Colitis (GETECCU) has just launched this prospective study, which will recruit 350 patients with an incidental diagnosis of IBD during colorectal cancer screening at 25 sites in Spain and compare them with two control cohorts (ClinicalTrials.gov: NCT05698745). Apart from describing the clinical, endoscopic and microscopic characteristics of these patients, the study will combine this information with multi-omic data including transcriptomic, proteomic, genetic, microbiome, serological and single-cell RNA sequencing data. The results from this study will provide detailed information about the disease expansion phase and, particularly, about the prognosis and potential disease stratification of subjects who will develop symptomatic disease and more aggressive phenotypes.
Main limitations of current evidence and looking into the future
Despite international consortiums and the available datasets, there are still some areas that will need to be clarified regarding the preclinical period. The main current limitation is the paucity of reports describing biomarkers and immune processes involved in the development of UC. Most data come from the GEM cohort and the IBD Character initiative that have demonstrated some faecal and circulating proteomic signatures in this setting [11, 14]. Another weakness of currently available reports is the scarcity of data on multi-omic analysis combining different techniques, which would help in understanding how all the layers involved in disease pathogenesis interact [18, 19]. Also, samples from different sources and tissues will increase our possibilities of finding new biomarkers and the triggers, in particular during the disease initiation and expansion phases. Nevertheless, the available evidence is of great value and is expected to drive further research that will change our understanding of IBD in the near future.
In conclusion, evaluation of the events preceding IBD diagnosis is an evolving and exciting area of research, where recent data have shown that the subclinical inflammatory process is detectable many years before diagnosis, similar to other immune-mediated inflammatory diseases. Research in this field will help us in multiple ways, including identification of individuals at risk, stratification according to risk of disease progression, further improvement of the beneficial effect of disease intervention strategies and even development of disease prevention approaches.
References
- Jayasooriya N, Baillie S, Blackwell J, et al. Systematic review with meta-analysis: Time to diagnosis and the impact of delayed diagnosis on clinical outcomes in inflammatory bowel disease. Aliment Pharmacol Ther 2023;57:635–52.
- Torres J, Halfvarson J, Rodriguez-Lago I, et al. Results of the Seventh Scientific Workshop of ECCO: Precision medicine in IBD – Prediction and prevention of inflammatory bowel disease. J Crohns Colitis 2021;15:1443–54.
- Agrawal M, Sabino J, Frias-Gomes C, et al. Early life exposures and the risk of inflammatory bowel disease: Systematic review and meta-analyses. EClinicalMedicine 2021;36:100884.
- Torres J, Petralia F, Sato T, et al. Serum biomarkers identify patients who will develop inflammatory bowel diseases up to 5 years before diagnosis. Gastroenterology 2020;159:96–104.
- Rodriguez-Lago I, Aguirre U, Ramirez de la Piscina P, et al. Subclinical bowel inflammation increases healthcare resources utilization and steroid use before diagnosis of inflammatory bowel disease. United European Gastroenterol J 2023;11:9–18.
- Bonfils L, Sandri AK, Poulsen GJ, et al. Medication-wide study: Exploring medication use ten years prior to a diagnosis of inflammatory bowel disease. Am J Gastroenterol 2023. Aug 10. doi: 10.14309/ajg.0000000000002399. Online ahead of print.
- Livanos AE, Dunn A, Fischer J, et al. Anti-integrin alphavbeta6 autoantibodies are a novel biomarker that antedate ulcerative colitis. Gastroenterology 2023;164:619–29.
- Choung RS, Petralia F, Torres J, et al. Preclinical serological signatures are associated with complicated Crohn's disease phenotype at diagnosis. Clin Gastroenterol Hepatol. 2023. Feb 12. doi: 10.1016/j.cgh.2023.01.033. Online ahead of print.
- Mortha A, Remark R, Del Valle DM, et al. Neutralizing anti-granulocyte macrophage-colony stimulating factor autoantibodies recognize post-translational glycosylations on granulocyte macrophage-colony stimulating factor years before diagnosis and predict complicated Crohn's disease. Gastroenterology 2022;163:659–70.
- Leibovitzh H, Lee SH, Raygoza Garay JA, et al. Immune response and barrier dysfunction-related proteomic signatures in preclinical phase of Crohn's disease highlight earliest events of pathogenesis. Gut 2023;72:1462–71.
- Bergemalm D, Andersson E, Hultdin J, et al. Systemic Inflammation in Preclinical Ulcerative Colitis. Gastroenterology 2021;161(5):1526–39 e9.
- Hua X, Ungaro RC, Petrick LM, et al. Inflammatory bowel disease is associated with prediagnostic perturbances in metabolic pathways. Gastroenterology 2023;164:147–50 e2.
- Brand EC, Klaassen MAY, Gacesa R, et al. Healthy cotwins share gut microbiome signatures with their inflammatory bowel disease twins and unrelated patients. Gastroenterology 2021;160:1970–85.
- alipeau HJ, Caminero A, Turpin W, et al. Novel fecal biomarkers that precede clinical diagnosis of ulcerative colitis. Gastroenterology 2021;160:1532–45.
- Rodriguez-Lago I, Merino O, Azagra I, et al. Characteristics and progression of preclinical inflammatory bowel disease. Clin Gastroenterol Hepatol 2018;16:1459–66.
- Rodriguez-Lago I, Ramirez C, Merino O, et al. Early microscopic findings in preclinical inflammatory bowel disease. Dig Liver Dis 2020;52:1467–72.
- Jess T, Vestergaard MV, Iversen AT, Allin KH. Undiagnosed inflammatory bowel disease among individuals undergoing colorectal cancer screening: a nationwide Danish cohort study 2014-2018. Gut 2023;72:214–6.
- Agrawal M, Allin KH, Petralia F, Colombel JF, Jess T. Multiomics to elucidate inflammatory bowel disease risk factors and pathways. Nat Rev Gastroenterol Hepatol 2022;19:399–409.
- Rodriguez-Lago I, Blackwell J, Mateos B, Marigorta UM, Barreiro-de Acosta M, Pollok R. Recent advances and potential multi-omics approaches in the early phases of inflammatory bowel disease. J Clin Med 2023;12(10):3418.
- Porter CK, Riddle MS, Gutierrez RL, et al. Cohort profile of the PRoteomic Evaluation and Discovery in an IBD Cohort of Tri-service Subjects (PREDICTS) study: Rationale, organization, design, and baseline characteristics. Contemp Clin Trials Commun 2019;14:100345.
- Turpin W, Lee SH, Raygoza Garay JA, et al. Increased intestinal permeability is associated with later development of Crohn's disease. Gastroenterology 2020;159:2092–100 e5.
- Leibovitzh H, Lee SH, Xue M, et al. Altered gut microbiome composition and function are associated with gut barrier dysfunction in healthy relatives of patients with Crohn's disease. Gastroenterology 2022;163:1364–76 e10.
- Lee SH, Turpin W, Espin-Garcia O, et al. Anti-microbial antibody response is associated with future onset of Crohn's disease independent of biomarkers of altered gut barrier function, subclinical inflammation, and genetic risk. Gastroenterology 2021;161:1540–51
- Blackwell J, Saxena S, Jayasooriya N, et al. Prevalence and duration of gastrointestinal symptoms before diagnosis of inflammatory bowel disease and predictors of timely specialist review: a population-based study. J Crohns Colitis 2020;15:203–2011.
- Blackwell J, Saxena S, Petersen I, et al. Depression in individuals who subsequently develop inflammatory bowel disease: a population-based nested case-control study. Gut 2021;70:1642–8.
- Faye AS, Allin KH, Iversen AT, et al. Antibiotic use as a risk factor for inflammatory bowel disease across the ages: a population-based cohort study. Gut 2023;72:663–70.
- Bisgaard TH, Poulsen G, Allin KH, Keefer L, Ananthakrishnan AN, Jess T. Longitudinal trajectories of anxiety, depression, and bipolar disorder in inflammatory bowel disease: a population-based cohort study. EClinicalMedicine 2023;59:101986.
