Microbial mechanisms of action of exclusive enteral nutrition in Crohn's Disease: Cause or effect?
Vaios Svolos, D-ECCO Committee Member
 Vaios Svolos Vaios Svolos © ECCO |
Exclusive enteral nutrition (EEN) is a prescribed liquid diet providing 100% of energy intake while excluding all other foods and drinks. It uses polymeric or elemental formulas, which are equally effective, though polymeric formulas are more palatable and better tolerated over 6–8 weeks. EEN is established as an effective therapy for Crohn's Disease (CD) that induces remission in up to 80% of patients [1, 2]. The clinical benefits of EEN include (a) the reduction of inflammation, with decreases in both blood and gut inflammatory markers and induction of mucosal healing, and (b) the improvement of clinical and physical health, with promotion of clinical remission and enhancement of muscle mass and nutritional status [3]. Studies have shown that EEN induces significant changes in the microbiome; although these changes are implicated in each mechanism of action, further research is needed to provide a better understanding of diet–microbiome interactions in CD [4, 5]. In the present article, the Bradford Hill criteria [6], a set of principles used to determine causal relationships between an exposure and a disease, are employed as a framework to analyse the EEN–microbiome interactions in CD. These criteria include factors such as strength, consistency, temporality and biological plausibility [6].
Criterion 1. Strength: strong relationship between variables. A significant decrease in bacterial diversity during treatment with EEN has been previously described within targeted and untargeted microbiome analysis studies. It is important to note that such paradoxical associations of disease improvement and induction of dysbiosis are of greater magnitude and strength among patients with a better disease response to EEN [7, 8].
Criterion 2. Experiment: change in cause = change in effect. The hypothesis of a causal relationship between microbiome changes and EEN clinical efficacy is further supported by the rebound of the gut microbiome to pre-treatment community characteristics after re-introduction of food [2, 8], and this rebound is mirrored by faecal calprotectin elevation post EEN cessation [9].
Criterion 3. Biological gradient: dose-response, more exposure leads to more outcome. Greater adherence to EEN correlates not only with better clinical outcomes, with higher volumes being significantly more efficacious, but also with stronger suppression of both microbiome composition and metabolic activity [10–12].
Criterion 4. Temporality: the cause must precede the outcome. Given the rapid inflammatory amelioration during EEN, with studies describing symptomatic and inflammation benefits within two weeks of initiation of treatment, it is important to confirm that the microbiome effect precedes the clinical benefit. In this context, studies have shown that microbial and metabolite changes are apparent within 7 days after EEN initiation [13].
Criterion 5. Consistency: the relationship is consistent in different studies/populations. Recent reviews have found that the suppressive microbial effects of EEN have been consistently described by different studies and within different populations, including paediatric and adult patients [3, 5].
Criterion 6. Coherence: the relationship is consistent with similar knowledge. In agreement with the apparent role of microbiome shifts during EEN therapy, a recent study has found that certain pre-treatment metabolic and microbial signatures are predictors of response to treatment with EEN [14].
Criterion 7. Plausibility: there is biological rationale for the relationship. The biological rationale for the discussed associations is provided by human genome studies identifying genetic variants that alter the intestinal microbiome and are associated with the development of IBD [15]. Further supportive evidence is provided by studies of animal models which have revealed that a germ-free state prevents the development of ileal disease, the pathogenesis of which resembles that of CD [16].
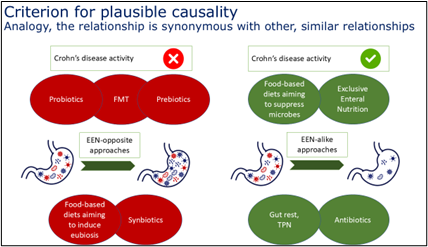
Criterion 8. Analogy: the relationship is synonymous with other, similar relationships. The relationship between EEN clinical benefit and microbiome suppression is synonymous with other similar relationships: (a) antibiotics which suppress the microbiome have shown some success in improving certain types of CD, (b) probiotics, prebiotics and synbiotics intended to modulate and enhance the microbiota lack efficacy in the treatment of CD, (c) faecal microbiota transplantation (FMT) is effective in conditions like C. difficile infections but has not been successful for CD and (d) food-based diets that aim to suppress gut microbial activity and diversity share similarities with EEN in targeting microbiome changes and also have similar benefits with regard to disease activity [1–4, 13]. These analogies highlight both complementary and contrasting approaches, emphasising EEN’s mechanisms of action.
Copyright of the graph © Vaios Svolos
The effectiveness of EEN in CD largely stems from its ability to manipulate the gut microbiome. By reducing inflammation and promoting mucosal healing, EEN provides a multifaceted therapeutic benefit. While further research is needed to fully elucidate the precise microbial changes and downstream effects, the central role of microbiota manipulation is increasingly clear. Current understanding attributes EEN's efficacy primarily to the suppression of gut microbiome activity. Using the Bradford-Hill criteria and analogous therapeutic comparisons, EEN’s mechanisms provide a framework to uncover additional pathways and improve the management of CD.
References
- Halmos EP, Godny L, Vanderstappen J, Sarbagili-Shabat C, Svolos V. Role of diet in prevention versus treatment of Crohn's disease and ulcerative colitis. Frontline Gastroenterol 2024;15:247–57.
- Gerasimidis K, Godny L, Sigall-Boneh R, Svolos V, Wall C, Halmos E. Current recommendations on the role of diet in the aetiology and management of IBD. Frontline Gastroenterol 2022;13:160–7.
- Gkikas K, Svolos V, Hansen R, Russell RK, Gerasimidis K. Take-home messages from 20 years of progress in dietary therapy of inflammatory bowel disease. Ann Nutr Metab 2023;79:476–84./li>
- Gerasimidis K, Gkikas K, Stewart C, Neelis E, Svolos V. Microbiome and paediatric gut diseases. Arch Dis Child 2022;107:784–9.
- Svolos V, Gkikas K, Gerasimidis K. Diet and gut microbiota manipulation for the management of Crohn's disease and ulcerative colitis. Proc Nutr Soc 2021:1–15.
- Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965;58:295–300.
- Quince C, Ijaz UZ, Loman N, et al. Extensive modulation of the fecal metagenome in children with Crohn's disease during exclusive enteral nutrition. Am J Gastroenterol 2015;110:1718–29; quiz 1730.
- Gerasimidis K, Bertz M, Hanske L, et al. Decline in presumptively protective gut bacterial species and metabolites are paradoxically associated with disease improvement in pediatric Crohn's disease during enteral nutrition. Inflamm Bowel Dis 2014;20:861–71.
- Logan M, Clark CM, Ijaz UZ, et al. The reduction of faecal calprotectin during exclusive enteral nutrition is lost rapidly after food re-introduction. Aliment Pharmacol Ther 2019;50:664–74.
- Jatkowska A, Gkikas K, Nichols B, et al. Dose-dependent effects of enteral nutrition on the faecal microbiota and short chain fatty acids. Clin Nutr 2024;43:1200–7.
- Jatkowska A, White B, Nichols B, et al. Development and validation of the Glasgow Exclusive Enteral Nutrition Index of Compliance. J Crohns Colitis 2023;17:1426–35.
- McKirdy S, Russell RK, Svolos V, et al. The impact of compliance during exclusive enteral nutrition on faecal calprotectin in children with Crohn disease. J Pediatr Gastroenterol Nutr 2022;74:801–4.
- Svolos V, Hansen R, Nichols B, et al. Treatment of active Crohn's disease with an ordinary food-based diet that replicates exclusive enteral nutrition. Gastroenterology 2019;156:1354–67.e1356.
- Nichols B, Briola A, Logan M, et al. Gut metabolome and microbiota signatures predict response to treatment with exclusive enteral nutrition in a prospective study in children with active Crohn's disease. Am J Clin Nutr 2024;119:885–95.
- Cohen LJ, Cho JH, Gevers D, Chu H. Genetic factors and the intestinal microbiome guide development of microbe-based therapies for inflammatory bowel diseases. Gastroenterology 2019;156:2174–89.
- aurog JD, Richardson JA, Croft JT, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med 1994;180:2359–64.