ECCO News Associate Editors’ Summary of the 2019 Y-ECCO Abstract Awards and Top 10 DOPs
Nuha Yassin and Ignacio Catalán-Serra, ECCO News Associate Editors
 Nuha Yassin © ECCO |
 Ignacio Catalán-Serra © Ignacio Catalán-Serra |
For this second issue of ECCO News in 2019, we would like to continue to review the excellent scientific content presented at the recent annual ECCO´19 annual meeting held in Copenhagen.
We would like to focus our attention this time on the contribution of the Y-ECCO Members, summarizing the five Y-ECCO Abstract Awards for this year, as well as to provide a comprehensive review of the ten awarded digital oral presentations (DOP).
We hope that this representation of high-quality research combining epidemiological, basic and clinical aspects of inflammatory bowel disease is of interest for a wide range of ECCO Members and helps encourage and inspire future innovative work.
Summary of Y-ECCO Abstract Awards
OP 15. Cost analysis in a prospective European population-based inception cohort: is there a cost-saving effect of biological therapy?
Johan Burisch, Frederikssund, Denmark
Updated epidemiological studies in the era of biologics in Inflammatory Bowel Disease (IBD) are of key importance for clinicians, researchers and health authorities.
Burisch et al. presented the results from the first prospective analysis of healthcare costs in patients with IBD in the era of biological treatments in Europe. The Epi-IBD cohort included 1362 IBD patients diagnosed from 31 centres in 20 European countries (Western Europe: 1104; Eastern Europe: 258) from 2010, with 5 years of follow-up. Of them, 52% had Ulcerative Colitis (UC), 37% Crohn’s Disease (CD) and 11% IBD unclassified (IBD-u).
Mean total expenditures per patient-year (PY) for both CD and UC are summarised in Tables 1 and 2.
| PY1 | PY2 | PY3 | PY4 | PY5 | |
|---|---|---|---|---|---|
| Total expenditure | 5579€ | 1820€ | 1714€ | 1907€ | 1669€ |
| Biological therapy (%) | 11 | 46 | 51 | 48 | 55 |
| Other IBD-related medication (%) | 5 | 13 | 11 | 11 | 12 |
| Hospitalisation (%) | 20 | 14 | 11 | 11 | 6 |
| Diagnostic procedures (%) | 34 | 17 | 11 | 12 | 10 |
| Surgery (%) | 30 | 9 | 16 | 18 | 17 |
Table 1. Mean total expenditure (€/patient) and proportion of expenditure spent on different categories in patients with CD.
| PY1 | PY2 | PY3 | PY4 | PY5 | |
|---|---|---|---|---|---|
| Total expenditure | 3612€ | 1421€ | 810€ | 983€ | 674€ |
| Biological therapy (%) | 2 | 7 | 20 | 19 | 25 |
| Other IBD-related medication (%) | 15 | 23 | 29 | 21 | 26 |
| Hospitalisation (%) | 35 | 29 | 21 | 33 | 17 |
| Diagnostic procedures (%) | 38 | 20 | 20 | 19 | 19 |
| Surgery (%) | 10 | 21 | 10 | 8 | 13 |
Table 2. Mean total expenditure (€/patient) and proportion of expenditure spent on different categories in patients with UC.
In both Eastern and Western Europe, total annual costs were highest in PY1 and then decreased, while the expenditure on biological therapy increased in this time period in both regions. The authors found that this increase was paralleled by a steady decrease in costs of non-biological treatment, hospitalisation and surgery. In a regression analysis of the data, patients with complicated disease phenotypes (penetrating CD or extensive UC), as well as patients aged ≥40 years, engendered higher costs.
This large European cost analysis confirms a cost-saving effect of biological medications, despite their high cost, as a result of reduced expenditure on standard medical treatments, surgery and hospitalisation. In addition, it provides important data on the current costs over different countries in Europe (Eastern and Western) and how patient characteristics impact healthcare costs, with potential translation to health care strategies.
OP 29. OST2+/IL-33 responsive cells promote tumorigenesis in colitis-associated colorectal cancer
Loris Riccardo Lopetuso, Rome, Italy
The incidence of colorectal carcinoma (CRC) in long-standing IBD is an issue of concern for clinicians, especially in UC patients and older populations.
This study aimed to explore the role of the IL33 pathway and its receptor, ST2, in inflammation-driven tumorigenesis leading to CRC using a mouse model of colitis-associated CRC – the azoxymethane (AOM)/dextran sodium sulphate (DSS) model.
C57/BL6 wild-type (WT), IL-33 KO, ST2 KO and CD73 KO mice were treated with AOM/DSS. Disease activity index (DAI) and endoscopic/histological evaluation of colons were performed. The authors studied full-thickness colons for IL-33 and ST2 (IHC, ISH, qPCR and mRNA expression) and used flow cytometry (FACS) analysis on cell suspensions from resected, isolated polyps. In addition, qPCR for Vimentin, Desmin, αSMA, CD34, CD31 and CD73 was performed on sorted cells. IL-33, ST2L and sST2 mRNA transcripts were significantly elevated in AOM/DSS-treated WT mice vs controls.
IL-33 was localised in the colonic epithelium in treated WT mice and ST2 staining was localised to the intestinal epithelium immediately adjacent to tumours, while little to no staining was present for either IL-33 or ST2 in controls.
FACS analysis showed a distinct population of CD45+ haematopoietic cells consisting of CD3/CD8+ cytotoxic T cells (CTLs), CD19+ B-lymphocytes and CD11b+CD11c- and CD11b+CD11c+ myeloid cells. ST2 was mainly expressed by CTLs and CD11b+CD11c- and CD11b+CD11c+ myeloid cells. At qPCR, CD45-ST2+ and CD45+ST2+ cells expressed significantly elevated levels of CD73 in comparison with ST2- cells.
Colonoscopy revealed a significantly decreased polyp number and size in IL-33, ST2 KO and CD73KO mice vs WT mice. All KO mice showed a higher degree of mucosal inflammation, which the investigators attributed to deficient IL-33 signalling.
Overall, these results suggest that the IL-33/ST2 axis promotes tumorigenesis in colitis-associated CRC through the activation of CD73, opening the way for new potential therapeutic targets for inflammation-driven tumorigenesis in IBD.
OP 32. A novel mechanism of colonic epithelial-T-cell cross-talk is dysregulated in IBD
Robin J. Dart, London, United Kingdom
There is increasing evidence on the key role of intraepithelial T cells and, more specifically, γδ T cells, in the pathogenesis of IBD. γδ T cells are essential players in intestinal homeostasis owing to their roles in maintaining tissue integrity and acting as a first line of defence against infections.
In this interesting study, Dart et al., describe a novel axis by which epithelial cells maintain homeostasis of the γδ T cell compartment, which is frequently dysregulated in IBD.
Human colonic γδ cells are regulated by specific members of the butyrophilin-like (BTNL) protein family that are restricted to intestine epithelial cells. Using short-term whole gut explant culture from IBD and controls, the authors investigated whether such selective regulation of human colonic γδ cells by BTNL3/8 is perturbed in IBD and, if so, which factors are involved.
In most controls, co-culture of colonic lymphocytes with HEK293T cells co-transduced with BTNL3+8 resulted in profound TCR down-regulation in Vγ2/3/4+ cells. The expression of αΕβ7, which is a marker of epithelial residence, was also associated with TCR down-regulation responses to BTNL3+8 in those cells. However, many patients with IBD presented a significantly reduced αΕβ7 expression by Vγ2/3/4+ cells and a severe attenuation or loss of BTNL-dependent Vγ2/3/4 TCR down-regulation. αΕβ7- γδ T cells demonstrated an activated pro-inflammatory phenotype in comparison to quiescent αΕβ7+ γδ T cells. Moreover, the addition of pro-inflammatory cytokines IL-12 and IL-18 (but not IL-1β and IL-23) to the organ culture led to down-regulation of αΕβ7 on γδ T cells and a consequent attenuation of response to BTNLs.
The authors conclude that these novel data on the regulation of γδ T cells might have therapeutic consequences. These results support the use of IL-12 blockade in restoring this axis while the therapeutic blockade of αΕβ7 can disrupt this important axis in the human colon and may exacerbate disease, given the pro-inflammatory nature of αΕβ7- γδ T cells.
DOP38. A vedolizumab specific four-gene colonic signature accurately predicting future endoscopic remission in patients with inflammatory bowel disease
Bram Verstockt, Leuven, Belgium
There is an urge to find predictive markers to select the right therapeutic compound for individual IBD patients. In this study, Verstock et al. present a novel vedolizumab-specific predictive 4-gene expression signature which may guide treatment strategy in IBD patients with colonic involvement.
The authors collected inflamed colonic biopsies from 31 patients (20 UC, 11 CD) prior to initiation of vedolizumab and from 24 patients initiating anti-TNF therapy (15 UC, 9 CD).
RNA sequencing identified 44 genes (25 down, 19 up) that were significantly differently expressed between future vedolizumab remitters and non-remitters. Involved pathways studied included glucocorticoid receptor signalling, differential regulation of cytokines in intestinal epithelial cells, granulocyte adhesions and diapedesis. Using randomised generalised linear modelling (RGLM), the authors identified and validated a predictor for vedolizumab-induced endoscopic remission defined as absence of ulcerations at month 6 for CD and Mayo endoscopic sub-score ≤1 at week 14 for UC.
When using these 44 differentially expressed genes as input for the RGLM modelling, the authors identified a four-gene signature which could accurately differentiate remitters and non-remitters in both the discovery (accuracy 90.9%, p=0.02) and the validation (100%, p=0.006) set. In addition, this four-gene signature was able to accurately discriminate prospective future remitters in a publicly available microarray data set of 13 open-label vedolizumab-treated UC patients, while it could not predict anti-TNF-induced endoscopic remission, suggesting its specificity.
This vedolizumab-specific predictive gene expression signature may guide treatment strategies in IBD patients with colonic involvement. The identification of this kind of prospective specific predictive marker will contribute to the application of precision medicine for IBD.
DOP 44. Efficacy and safety of tacrolimus in ulcerative colitis: a nationwide, multi-centre study from GETECCU
Iago Rodriguez-Lago, Galdakao, Spain
Tacrolimus (TCR) is an immunosuppressive calcineurin inhibitor drug commonly used in the context of renal and liver transplantation that has been shown to be effective in refractory UC.
Rodriguez-Lago et al. presented a study evaluating the efficacy and safety of TCR for UC in clinical practice in Spain. The authors performed a retrospective, multi-centre study including 22 IBD units (between January 1999 and June 2018). A total of 58 adult patients with an established diagnosis of UC in whom oral TCR was prescribed were included.
The most common indications for TCR were steroid dependency (55%) and steroid-refractory disease (29%). Previous exposure to anti-TNF agents was observed in 71% (43% exposed to ≥2 anti-TNFs) and 22% to vedolizumab.
Partial Mayo score showed a statistically significant decrease after 3 months (clinical remission was achieved in 24%, while 36% were in partial clinical response). CRP levels showed statistically significant differences after 1, 3 and 6 months when compared with baseline. However, more than one-third of patients (35%) suffered adverse events related to the drug (40% tremor, 20% asthenia), and TCR had to be discontinued in up to 35% of the cases.
In the follow-up study, TCR was stopped in 81% of patients after a median of 14 months, with 47% of patients requiring a new immunomodulator, 28% hospitalisation and 33% colectomy.
The investigators conclude that tacrolimus offers a clinical benefit in medically refractory UC cases in the short term, but its long-term use might be limited by safety issues in clinical practice.
Summary of Top 10 Digital Oral Presentations
DOP 08. The regulatory landscape of intestinal cells investigating the transcriptional effect of autophagy impairment observed in Crohn’s disease using organoid and network biology approaches
Agatha Treveil, Norwich, United Kingdom
The use of systems biology and the different -omics has opened a new approach to the understanding of complex biological processes like the loss of the intestinal homeostasis involved in CD.
In this innovative work, Treveil et al. developed an integrative workflow to study regulatory pathways of intestinal cells (with a focus on Paneth cells), using organoids and network biology. The aim was to study potential master regulators of Paneth cells and to analyse the regulatory effect of autophagy impairment using a CD model.
To accomplish this, the authors performed transcriptomics analysis on differentiated organoids derived from normal mice and mice deficient in the autophagy-related protein Atg16l1. The analysed RNAs (mRNAs, miRNAs and long non-coding RNAs) were contextualised by linking them together into a unified regulatory network generated from published databases.
With this approach, the authors were able to identify regulators potentially contributing to Paneth cell-specific functions, such as four nuclear hormone receptors with links to IBD, immunity and autophagy (i.e. Vdr, Rxra, Nr1d1 and Nr3c1), and were able to describe the effect of autophagy impairment on the regulatory landscape of Paneth cell function.
This work shows that the application of a systemic approach in a cell-type specific context could help disentangle multifactorial mechanisms in CD, with potential clinical use.
DOP10. Serum N-glycomic biomarkers predict treatment escalation in inflammatory bowel disease
Archana Shubhakar, Abingdon, United Kingdom
There is a need to find simple serological markers that can predict response to the different drugs used in IBD. Shubhakar et al. studied serum N-glycan signatures as predictors of the need for treatment escalation. Serum samples from 227 IBD patients and 195 controls from Edinburgh (UK) were analysed by automated high-throughput fluorescent labelling of total serum N-glycans (TSNG) using ultra-high-performance liquid chromatography.
The glycomics biomarkers for treatment escalation (to anti-TNF, biologics and surgery) gave a hazard ratio (HR) of 23.73 (p=6.81 × 10−6) for CD and an HR of 30.83 (p=1.88 × 10−4) for UC, with a composite marker for all IBD patients achieving an HR of 25.91 (p=1.12 × 10–12). These findings were replicated in a cohort of 49 patients (including 15 requiring treatment escalation) recruited in Örebro (Sweden) to validate the escalation biomarker, with an HR of 5.07 (p=1.14 × 10–5).
The authors conclude that these serum N-glycan signatures are accurate in predicting the need for treatment escalation in IBD patients. This approach can contribute to the implementation of precision medicine in IBD.
DOP 22. UC-related and segment-specific properties of patient-derived colonic organoids
Kohei Suzuki, Tokyo, Japan
Suzuki et al. studied colonic organoids derived from IBD patients and controls to investigate to what extent the patient-derived organoids generated from different colonic segments exhibit disease-specific phenotypes.
A total of 55 colonic organoids were established from UC or CD patients in remission and controls. The authors analysed the colonic segment-specific gene expression by microarray analysis, and colonic stem cell-specific gene expression was examined at the single-cell level by microfluid-based multiplex qPCR analysis.
The analysis of organoids established from different colonic segments of the same patient could identify candidate segment-specific genes of the ascending colon (15 genes), transverse colon (5 genes), sigmoid colon (3 genes) and rectum (5 genes). However, single-cell gene expression data of 12 representative intestinal stem cell marker genes revealed an indistinguishable expression pattern in segment-matched organoids of UC and controls.
While the proliferation/growth efficiency profile of non-IBD patient-derived organoids from the ascending colon and the rectum was similar, UC patient-derived organoids clearly showed a segment-specific proliferation pattern (the ascending colon generally exhibited over twofold higher proliferation efficiency compared with the rectum).
These results suggest colonic segment-specific modification of colonocyte function in UC patients and contribute to our understanding of the colonic pathophysiological changes in the context of IBD.
DOP36 Gut microbial variations in patients with quiescent Crohn’s disease predict subsequent disease flare
Yael Haberman, Tel Hashomer, Israel
In this observational study, the researchers aimed to elucidate whether changes in the microbiome in quiescent CD patients are instrumental in increasing the risk for flares and can precede or predict clinical relapse.
A cohort of patients with quiescent CD (45 cases, 217 samples) was followed-up over 2 years or until a clinical flare occurred. Clinical assessment, faecal calprotectin, faecal microbial characterisation using 16S rRNA Illumina and CRP were measured routinely every 3 months and by video capsule endoscopy every 6 months.
Of the 45 patients with quiescent CD, 12 (27%) flared during follow-up. Faecal microbiota study in this group showed a significantly reduced abundance of Christensenellaceae and S24.7, and an increased abundance of Gemellaceae in comparison to patients in remission. A composite ‘flare index’ summarising those microbial taxa was significantly higher in patients who subsequently flared than in those who remained in remission. Of note, higher individualised microbial instability in the quiescent phase was associated with a higher risk of subsequent flares (hazard ratio 11.32, 95% CI 3–42, p=0.0035) using two pre-flare samples. In addition, higher individualised microbial instability in the quiescent phase was associated with a higher risk of subsequent flares (hazard ratio 11.32, 95% CI 3–42, p=0.0035) using two pre-flare samples.
Machine learning was employed to prioritise microbial and clinical factors that discriminate between relapsers and non-relapsers in the quiescent phase, showing that the top contributing factors were the ‘flare index’ and the intra-personal microbial instability. The authors also note that clinical activity markers like CRP and calprotectin or treatment exposure were not within the top five contributing factors in the prediction model.
This work adds important information about how individualised microbial variations in quiescent CD can precede and predict future exacerbations.
DOP45 Adequate infliximab exposure during the induction phase is associated with early complete fistula response in patients with fistulizing Crohn’s disease: a post-hoc analysis of the ACCENT-2 trial
Niels Vande Casteele, La Jolla, USA
Vande Casteele et al. aimed to investigate IFX induction concentration cut-points associated with early post-induction complete response in patients with fistulising CD.
The authors assessed the association of IFX serum concentrations at weeks (W) 2, 6 and 14 with complete fistula response assessed at W14 (CFR14), defined as a complete absence of draining fistulas. Patients from the ACCENT-2 trial, which included 282 patients with active fistulising CD treated with IFX, were analysed.
The median (interquartile range, IQR) IFX concentrations were significantly higher in patients who achieved complete fistula response (CFR14) compared with those who did not, both at W6 [18.4 (12.7–27.8) μg/ml vs 15.2 (9.1–26.0) μg/ml; p=0.038] and W14 [6.4 (2.3–10.8) μg/ml vs 3.7 (1.5–7.3) μg/ml; p=0.001]. Notably, IFX cut-points of 13.9 μg/ml at W6 and 4.8 μg/ml at W14 were associated with CFR14 and a multivariable logistic regression analysis identified W14 IFX concentration as the only independent factor associated with CFR14.
This study shows that higher IFX concentrations during induction are associated with early complete fistula response in patients with fistulising CD and highlights the importance of achieving high IFX induction levels in this subset of patients to improve clinical results.
DOP47 Sustained remission in patients with moderate to severe ulcerative colitis: Results from the Phase 3 UNIFI maintenance study
Laurent Peyrin-Biroulet, Vandœuvre-lès-Nancy, France
This study presents new data on sustained remission through week 44 of patients included in the UNIFI randomised-withdrawal maintenance study evaluating the safety and efficacy of subcutaneous (SC) ustekinumab in patients with moderately to severely active Ulcerative Colitis (UC) who had responded to intravenous (IV) ustekinumab during induction.
In total, 523 patients who had responded to IV ustekinumab induction were randomly assigned in a 1:1:1 ratio to placebo SC, ustekinumab SC 90 mg q12w or ustekinumab SC 90 mg q8w. The proportions of patients in symptomatic remission and IBDQ remission were generally similar among the treatment groups at baseline of the maintenance study, but the proportion of patients with endoscopic healing at baseline was lower in the ustekinumab q8w group (32.4%) compared with the ustekinumab q12w (39.5%) and placebo groups (40.6%).
Results for sustained remission through week 44, as measured by partial Mayo remission, symptomatic remission, endoscopic healing and IBDQ remission, in patients who achieved clinical response 8 weeks after receiving ustekinumab IV induction therapy were presented in this table:
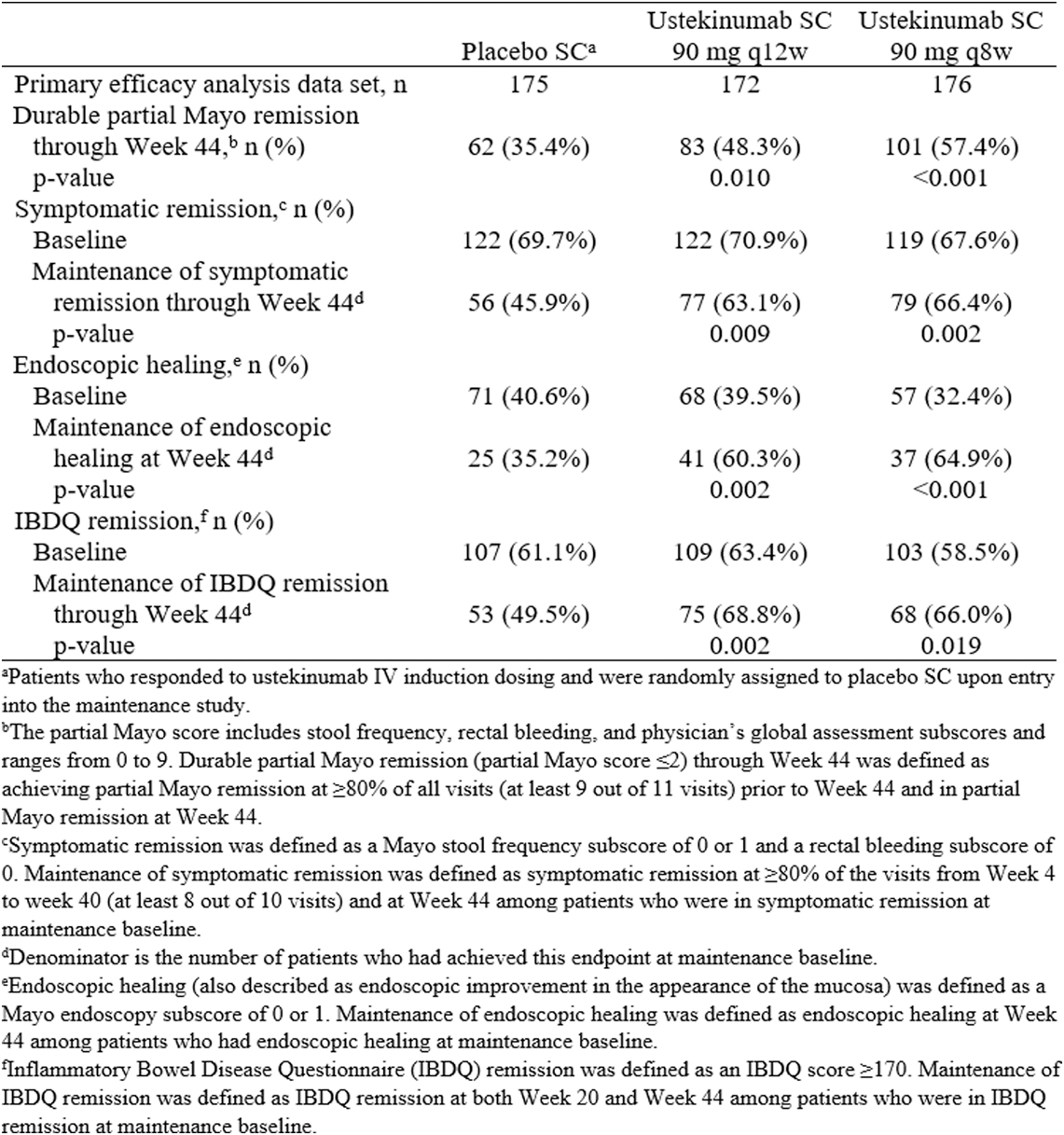
Significantly greater proportions of patients in the ustekinumab q8w and q12w groups compared with the placebo group maintained durable partial Mayo remission, symptomatic remission, IBDQ remission and endoscopic healing through week 44. The following figure shows the proportions of patients in the ustekinumab treatment groups and the placebo group who maintained partial Mayo remission:
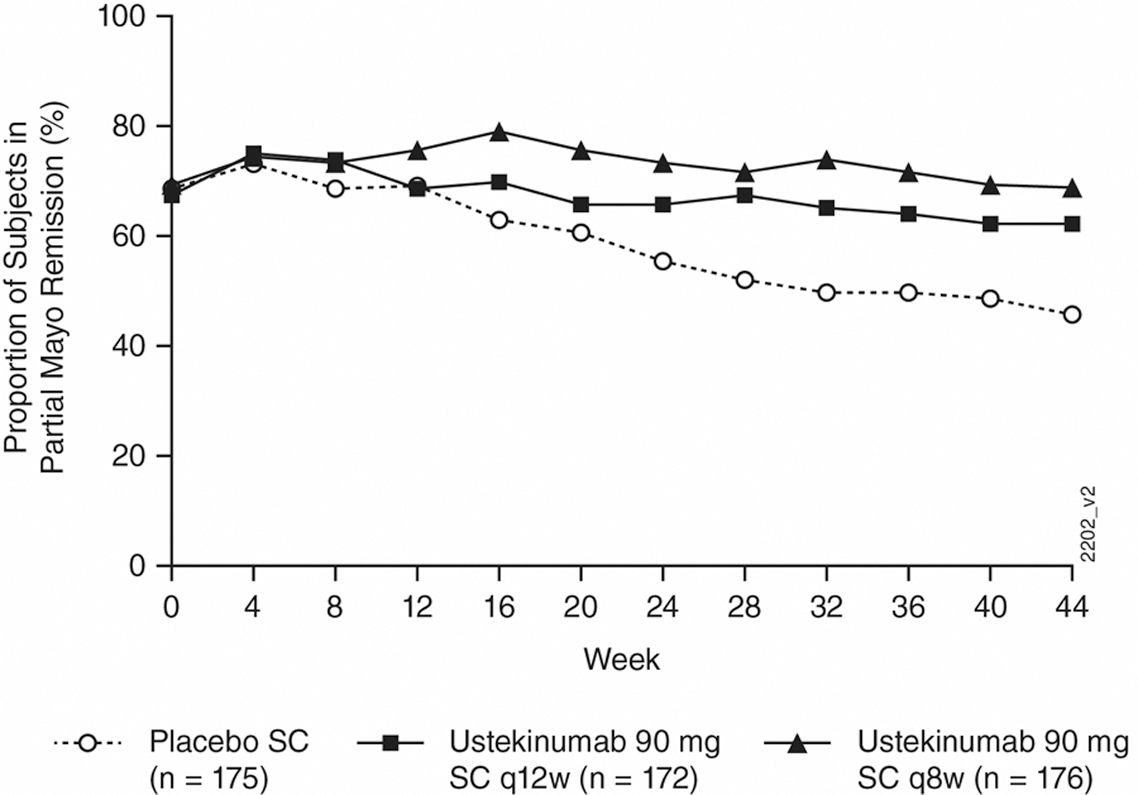
These results confirm that both doses of ustekinumab SC maintenance therapy sustained remission (measured by patient-reported symptoms and endoscopic and quality of life assessments) in patients with moderately to severely active UC at week 44.
DOP56 Dashboard driven vs. conventional dosing of infliximab in inflammatory bowel disease patients: the PRECISION trial
Anne Strik, Amsterdam, The Netherlands
Limited prospective data are available on the benefit of proactive dose adjustment based on serum IFX levels. Strik et al. presented the results of the PRECISION trial, which aims to investigate the efficacy of dashboard-driven IFX dosing in IBD patients during 1 year prospectively.
This multi-centre 1:1 randomised prospective trial included patients in clinical remission [Harvey–Bradshaw Index (HBI) ≤4 for CD or partial Mayo score (PM) ≤2 for UC] who were receiving IFX maintenance treatment. Patients in the precision dosing group (PG) received IFX dosing guided by a Bayesian pharmacokinetic model, aiming to achieve and maintain an IFX trough level (TL) of 3 µg/ml by treatment (de-)escalation as indicated by the dashboard. Patients in the control group (CG) continued to receive the IFX treatment regimen given prior to randomisation, without dose adaptation. The authors defined biochemical remission as a faecal calprotectin <250 µg/g and CRP <0.5 mg/l and clinical loss of response as an HBI >4 or a PM score >2 at two consecutive visits.
In total, 80 patients were included (66 CD and 14 UC). Per protocol analysis showed loss of clinical response in 14/39 (36%) patients in the CG compared with 4/32 (13%) patients in the PG (p=0.03). Three patients (7.5%) in the PG were considered failures because of re-opening of their perianal fistula after dose de-escalation to achieve a TL of 3 µg/ml. Time to relapse was evaluated using Kaplan-Meier analysis, as shown in this figure:
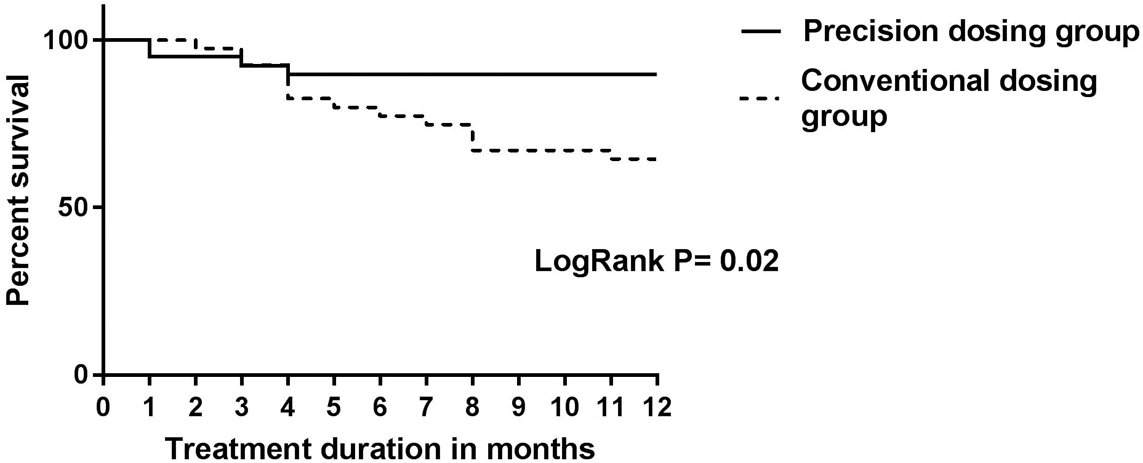
These results from the PRECISION study demonstrate a clinical benefit from personalised dosing in IBD patients in a prospective trial. Of note, de-escalating treatment to obtain an IFX TL of 3 µg/ml in patients with perianal disease resulted in fistulas re-opening, suggesting that higher TLs might be needed in this subpopulation.
DOP72 Increased risk of advanced neoplasia in inflammatory bowel disease patients with recurrent low-grade dysplasia
Michiel de Jong, Nijmegen, The Netherlands
The occurrence of low-grade dysplasia (LGD) is a major risk factor for the development of high-grade dysplasia (HGD) and colorectal cancer (CRC) in IBD patients, and intensified surveillance is generally recommended. In this study, the researchers aimed to assess how a second (recurrent) LGD finding impacts the long-term advanced neoplasia risk (HGD and/or CRC), compared to patients without subsequent dysplasia after initial LGD.
In total, 1215 IBD patients with colonic LGD and follow-up colonoscopy within 3 years from the Dutch nationwide network and registry of histo- and cytopathology were analysed (76.0% with UC, 17.6% with CD and 6.4% with IBD-u).
Mean time from initial LGD to first follow-up colonoscopy was 1.5 (±0.6) years in both groups, and a total of 259 patients (21.3%) had recurrent LGD within 3 years. Of them, 46 (17.8%) developed advanced neoplasia (31 CRC and 15 HGD) vs 10.9% of patients without recurrent LGD.
Patients with recurrent LGD had a higher cumulative advanced neoplasia incidence (HR 1.70; 95% CI 1.20–2.41; p=0.003), as shown by a Kaplan-Meier plot in this figure:
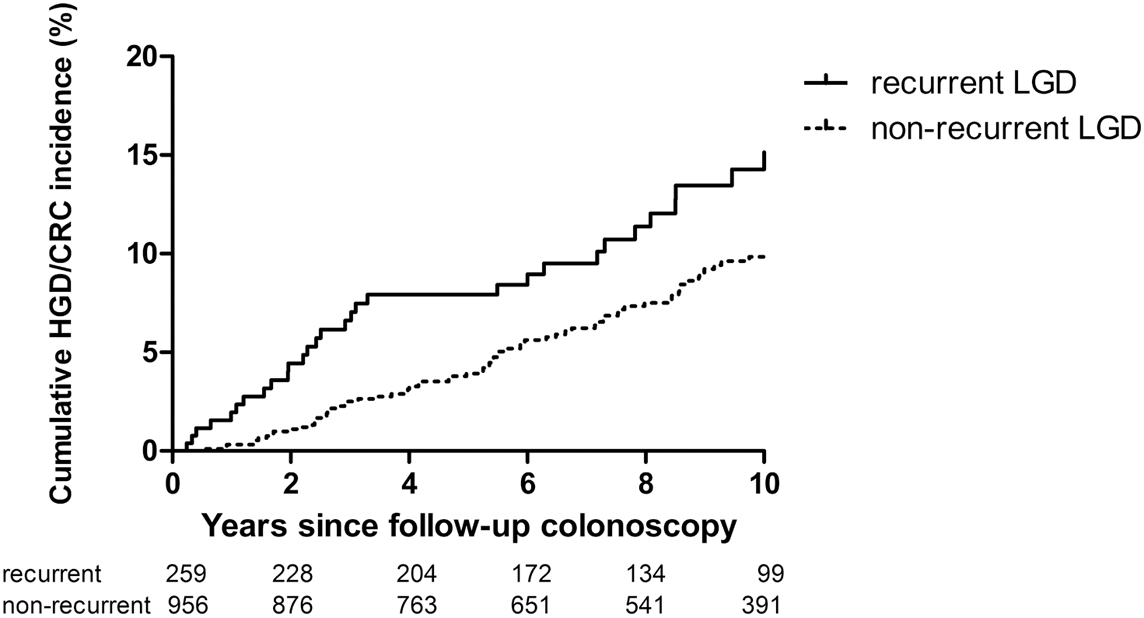
Further analysis showed that the cumulative advanced neoplasia incidence 2 years after follow-up surveillance colonoscopy was 4.4% in patients with recurrent LGD vs 1.4% in those without recurrence.
This study confirms that the presence of recurrent LGD at follow-up colonoscopy after initial LGD increased significantly the advanced neoplasia risk, supporting the current clinical recommendations.
DOP81 Utility of a simple blood test for mucosal healing monitoring is accurate in post-operative Crohn’s disease
Amy L. Hamilton, Melbourne, Australia
In this work, Hamilton et al. aimed to explore the utility of the Mucosal Healing Index (MHI), a computed score based on a serological panel of 13 markers, in the post-operative setting in CD patients.
The authors included patients from the Post-Operative Crohn Endoscopic Recurrence (POCER) study who underwent intestinal resection and colonoscopic assessment at 6 and 18 months and also provided serum (preoperatively and at 6, 12 and 18 months). Mucosal healing markers (Monitr™ panel; measuring CEACAM, VCAM, CRP, SAA, Ang-1, Ang-2, MMP-1, -2, -3, -9, EMMPRIN, TGF-α, IL-7) were used with proprietary calculation to derive the MHI. Endoscopic disease was assessed using Rutgeerts Score (i0–i4; recurrence ≥i2).
In total, 132 patients provided 439 samples for assessment (95 samples had matched serum and endoscopy at 6 months and 107 at 18 months). The median MHI was lower in those patients in remission at both 6 and 18 months (6 months: <i2 MHI 21.5 vs ≥i2 MHI 29.3, p=0.037; 18 months <i2 MHI 22.3 vs ≥i2 MHI 26.7, p=0.083). The correlation reported between the MHI and the Rutgeerts score at 6 and 18 months was significant but weak (r=0.24; p=0.0004).
The test performance of the MHI in post-operative CD recurrence is summarised in this table:
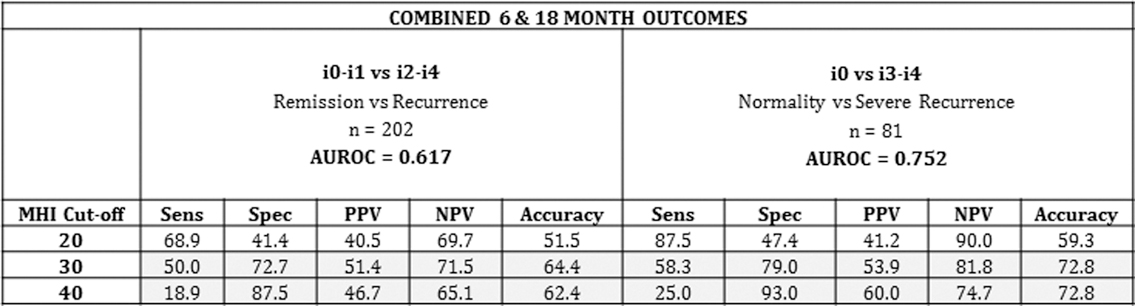
AUROC was 0.75 for discrimination between mucosal normality (i0) and severe recurrence (i3–i4) (95% CI 0.64–0.87) and 0.617 for discrimination between remission (i0–i1) and recurrence (i2–i4). The diagnostic accuracy of the different cut-offs was examined: an MHI cut-off of ≤20 had a sensitivity of 87.5% to rule out severe recurrence while a cut-off of 40 had a 93% specificity to rule it in.
The authors conclude that the non-invasive multi-marker serum test (MHI) has sufficient accuracy to be a useful adjunctive test for monitoring of post-operative CD recurrence.
DOP88 Thiopurine and allopurinol combination therapy and the risk of adverse outcomes and step-up medical therapy in inflammatory bowel disease patients: a nationwide Danish cohort study
Sandra Bohn Thomsen, Copenhagen, Denmark
The combination therapy of thiopurine and allopurinol has shown clinical benefit for the maintenance of remission in IBD patients, but the risk of adverse outcomes is unclear. In this study, Thomsen et al., using Danish registry data, aimed to study the outcomes in IBD patients treated with thiopurine and allopurinol combination therapy compared with those treated with thiopurine monotherapy.
The authors established a nationwide cohort of patients with IBD who had been prescribed thiopurine therapy between 1999 and 2014. The primary outcome was a composite of any adverse outcome or need for biological treatment: IBD-related hospitalisation, IBD-related surgery, biological therapy initiation or death, whichever came first. Poisson regression analyses were used to calculate incidence rate ratios (IRR) with 95% confidence intervals.
In total, 10,367 patients with IBD (CD=5484; UC=4883) were prescribed thiopurines, and of these 217 were exposed to allopurinol co-therapy. The authors observed 40 incident outcomes in patients exposed to allopurinol co-therapy among 129 person-years (PY) (IR=310.1 per 1000 PY) vs 4745 among 24,585 PY (IR=193.0 per 1000 PY) in patients exposed to thiopurine monotherapy. The adjusted IRR of an adverse outcome was not significantly different in the two groups of patients, nor did the results differ when analysed in strata by IBD subtype.
The authors conclude that IBD patients exposed to combination therapy with thiopurine and allopurinol did not have a statistically significant different risk of surgery, hospitalisation, biological therapy initiation or death when compared with IBD patients exposed to thiopurine monotherapy. The improvement in clinical remission with the combination therapy that was shown in previous studies was not confirmed in this study, since the results do not suggest an association with subsequent clinical outcomes.