Report on the 4th School for Clinical Trialists at ECCO'21
Laurent Beaugerie, ClinCom Member
 Laurent Beaugerie Laurent Beaugerie© ECCO |
This year’s 4th School for Clinical Trialists, organised by the ECCO Clinical Research Committee (ClinCom), offered an excellent opportunity to review all the key points of the balance between expected efficacy and potential risks of IBD drugs, in the contexts of both clinical trials and routine care.
In a preamble to the first session, dedicated to drug efficacy, Alissa Jane Walsh and Gemma Wakefield gave their points of view, as a gastroenterologist and a patient respectively, on patient expectations prior to entry into a trial. In brief, the motivations of patients are very diverse, depending in particular on personal disability and on previous good or bad experiences with other drugs. However, patients constantly appreciate the intensive and personalised care related to the particular context of clinical research. Of note, patient expectations can evolve throughout the trial.
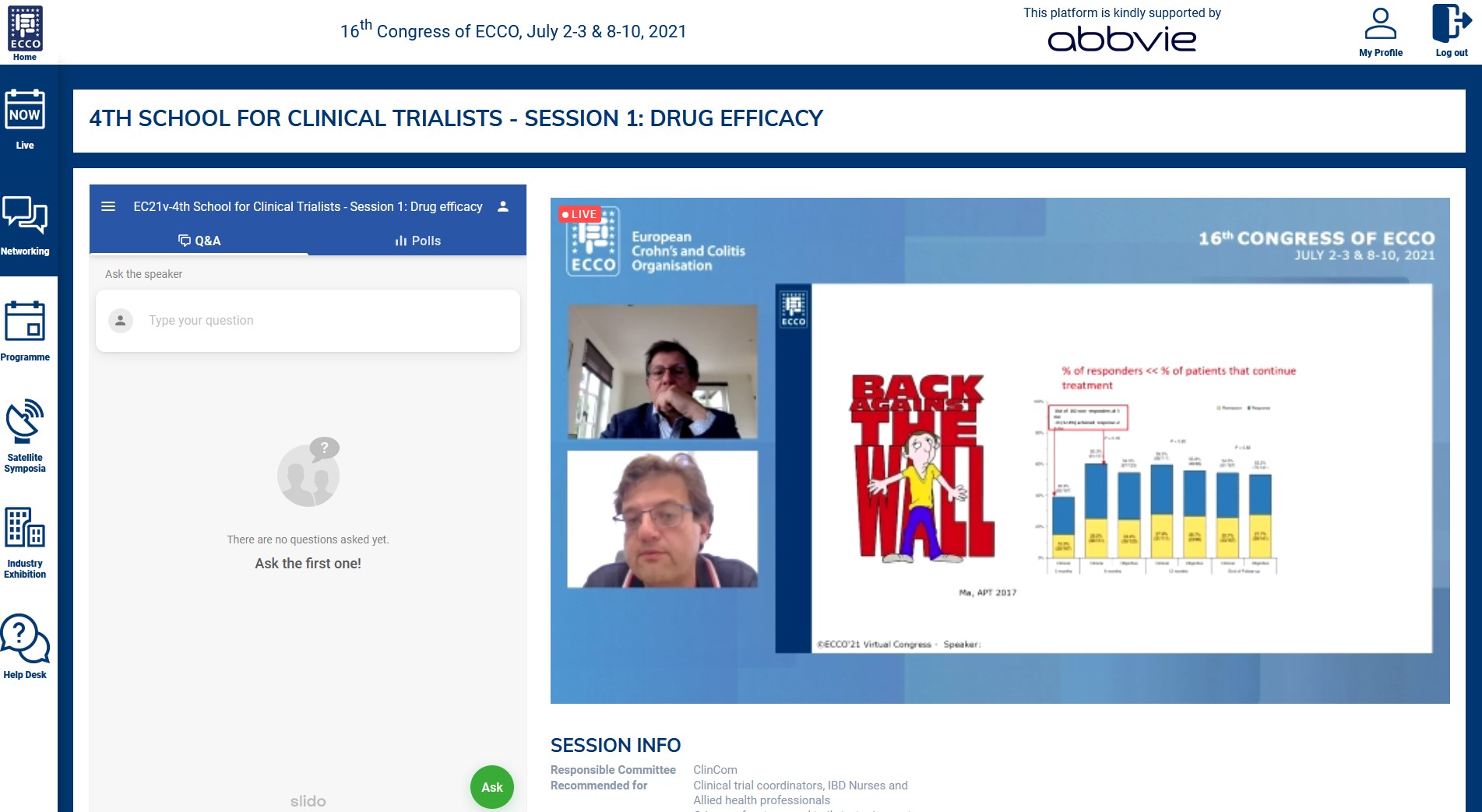
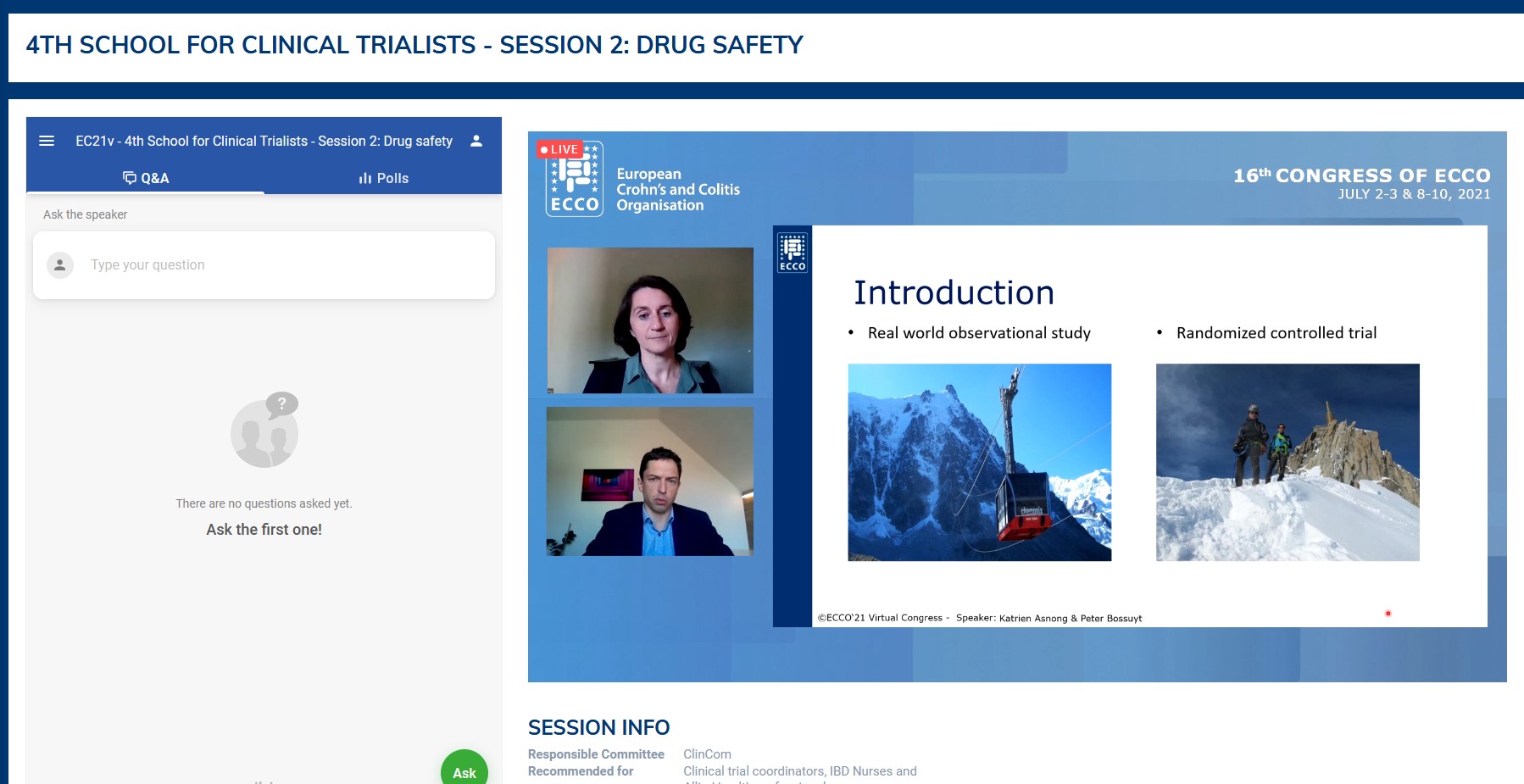
In a first tandem talk, Geert D’Haens and Uri Kopylov finally agreed that the ‘classical’ randomised controlled trials, although complicated to carry out and very time and money consuming, remain the only way to provide scientific proof of the effectiveness of a new IBD drug compared with placebo. But clinicians are increasingly waiting for randomised head-to-head comparisons to help them choose the best therapeutic options. In addition, many subgroups of patients with particular IBD phenotypes (proctitis in Ulcerative Colitis, upper digestive tract involvement in Crohn’s Disease) or vulnerabilities (older age, recent history of cancer) are not eligible for the trials. In these contexts, studies from real-world data are increasingly filling the gaps in knowledge, even if a high level of evidence is not always achieved. Depending on the questions asked, the efficacy data may derive from well-phenotyped huge IBD centre databases, from population-based medico-administrative databases or from dedicated investigator-initiative cohorts (I-CARE, UR-CARE).
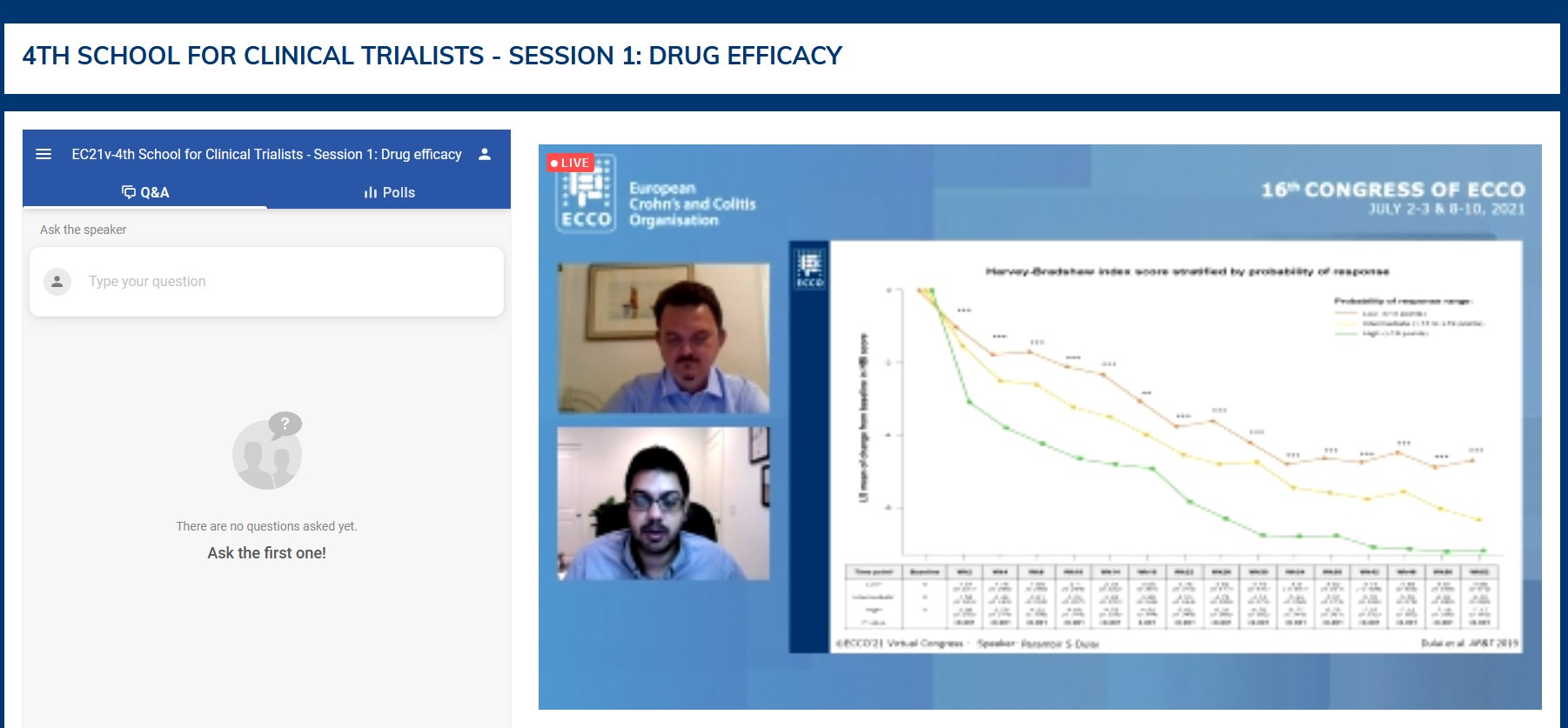
In a second tandem talk, Stefanos Bonovas and Parambir Dulai discussed the respective value of statistics, artificial intelligence and algorithms in guiding therapeutic choices. Starting with a limited number of potential clinical/biological predictors, classical statistical multivariate models are more appropriate than artificial intelligence in order to identify independent drivers of drug efficacy and then elaborate scores. These scores may in some cases be used routinely, providing subgroup ranges (rather than personalised numbers) of expected efficacy. The scores can also provide the rationale for stratification in randomised clinical trials and for elaboration of distinct pathways in therapeutic algorithms.
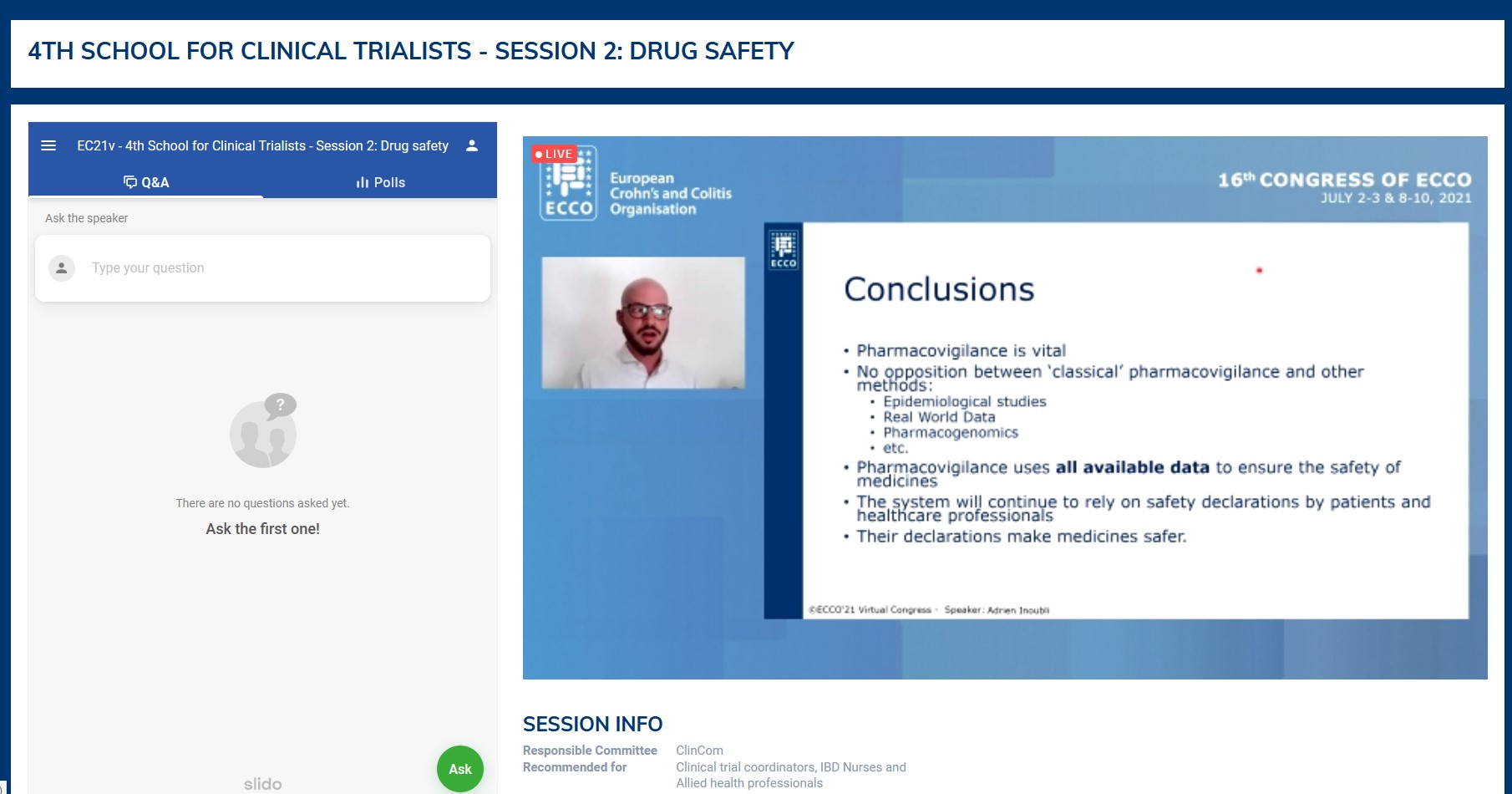
The second session was dedicated to drug safety assessment techniques and to communication of risks. Taking the example of unexpected adverse drug reactions to COVID vaccines, Adrien Inoubli brilliantly demonstrated that ‘classical’ pharmacovigilance is still extremely important through a three-step process. At the European level, spontaneous reports from patients and health care professionals are transmitted by pharmas and national competent authorities to the European Medicine Agency (EMA). Data are then centralised in a dedicated database (EudraVigilance) and analysed by the Pharmacovigilance Risk Assessment Committee (PRAC). Depending on the type, frequency and severity of events, specific actions are taken (prospective post-safety studies, restriction of drug use, etc.). Julien Kirchgesner then convinced us that pharmaco-epidemiology is essential to detect and confirm the causal link between a drug and a rare serious event. For this purpose, big medico-administrative databases, well-phenotyped electronic health records deriving from routine care and prospective investigator-initiated cohorts are the three major data sources, each having its strengths and limitations. In order to best approach the true causal role of drugs, the methodological quality of the adjustment for fixed and time-dependent confounders is crucial
The last tandem presentation was delivered by a senior clinical researcher (Peter Bossuyt) and an IBD Research Nurse (Katrien Asnong). With their experience in the field, they suggested tips and tricks on how best to communicate to patients the benefit-risk balance of IBD drugs. In brief, we should give priority to absolute numbers and visual representation of risks, and not hesitate to compare risk levels for drugs with those for other life events such as accidents. We should present nuanced written information and oral explanations that take maximum account of the patient's psychology, fears, expectations and previous experiences. Ultimately, after the provision of comprehensive evidence-based information, the emotional component of the patient’s preference should be respected.
The 4th School for Clinical Trialists was attended by 55 registrants from 16 countries and included physicians with different levels of experience in IBD as well as other health care professionals involved in clinical research and routine care management in the field of IBD.
Browse through some screenshots:
Pictures are subject to copyright © ECCO