Final Report, ECCO Grant for Margarita Papatheodoridi
Margarita Papatheodoridi, ECCO Grant Awardee
Deciphering the bioactive role of extracellular matrix fragments (matrikines) in Crohn's Disease (CD) fibrostenosis as potential therapeutic targets and biomarkers
 Margarita Papatheodoridi © Margarita Papatheodoridi |
Background & aim of research
To evaluate the in vitro effect of selected novel matrix-derived peptides (matrikines) that specifically appear in the intestinal tissue of patients with Crohn’s Disease (CD) fibrostenosis on primary human intestinal myofibroblasts (iMFBs).
Methodology used in the research
Primary human iMFBs were cultured in serum-free medium (SFM), complete medium, PDGF-bb 10 ng/mL or each of the matrikines at 10, 50 and 100 μg/mL for 24 hours. The effect of each matrikine on cell proliferation, gene expression and migration was assessed by BrdU, qPCR and Transwell assays, respectively. RNA sequencing data were analysed using the GSEA software.
Of the originally proposed experiments, co-cultures with HUVECs and macrophages were not completed due to insufficient time. However, data from RNA sequencing from the iMFB co-cultures were rich, suggesting possible interactions with those cell types, too, and this will be investigated in the near future.
Main findings/results of the research
iMFB proliferation and viability were tested with each of the 19 matrikines at three different doses (Table 1). Five peptides with the maximum effect on cell proliferation were selected for further experiments (Figure 1).
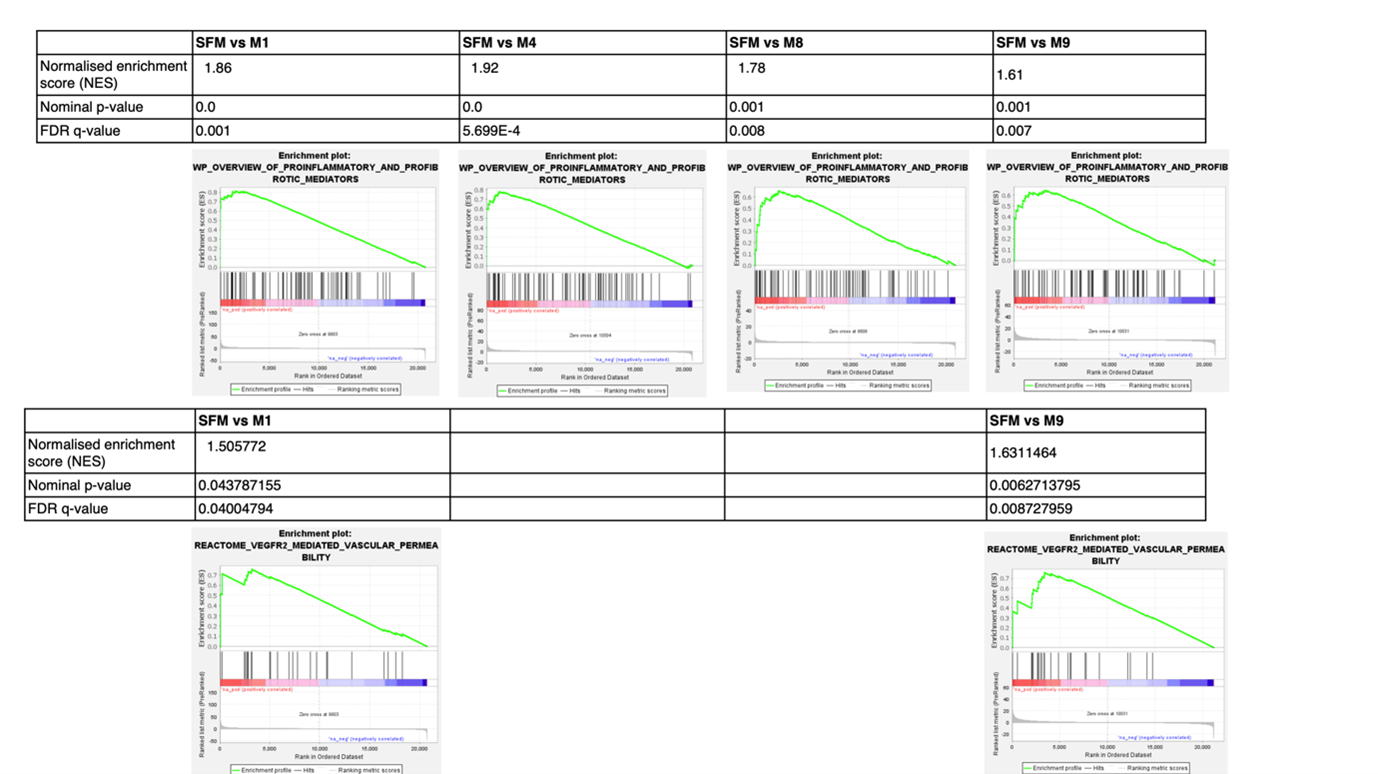
Different matrikines had various significant effects on gene expression, e.g. ACTA2 upregulation by M1 and M7; COL1A1 upregulation by M1, M7 or M8 (Figure 2). Furthermore, M1, M4 and M8 significantly induced iMFB migration (Figure 3). A total of 1948, 1024, 460, 570 and 2749 differentially expressed genes (DEGs) were identified in iMBFs treated with matrikines vs no treatment, showing enrichment in pathways related to “smooth muscle contraction”, “fibroblast growth factor”, “cell migration”, “collagen-elastin formation” and “ECM organisation”, most of which were also enriched by complete medium and/or PDGF-bb that served as positive controls. However, pathways related to “proinflammatory and profibrotic mediators” or “vascular permeability” were only enriched in iMFBs treated with the matrikine peptides (Figure 3).
In conclusion, a selection of newly discovered CD-specific matrikines induce activation of iMFBs as well as inflammatory response and endothelial changes, suggesting an important role, and therefore their potential value as biomarkers, in the progression of intestinal fibrostenosis.
Table 1. Effect of matrikines M1–M9 likely-to-be bioactive and N1–N10 unlikely-to-be bioactive on cell proliferation and viability of primary human iMFBs in vitro. Doses of 10, 50 and 100 μg/mL were tested for each peptide and comparisons were made with vehicle control (serum-free medium + DMSO 1.25%. Presence of effect in cell proliferation is reported if relative comparisons were statistically significant (p<0.05). N/s = non-significant
|
Matrikine |
Cell proliferation |
Cell viability |
||
|
|
Induction, yes or no (dose) |
Inhibition, yes or no (dose) |
Dose-dependent effect, yes or no |
|
|
M1 |
No |
Yes |
N/s |
|
|
M2 |
No |
Yes (100 μg/mL) |
Yes |
N/s |
|
M3 |
No |
Yes (100 μg/mL) |
Yes |
N/s |
|
M4 |
Yes (10 μg/mL) |
Yes (100 μg/mL) |
Yes |
N/s |
|
M5 |
Yes (50, 100 μg/mL) |
No |
No |
N/s at 50 μg/mL, decreased at 100 μg/mL |
|
M6 |
Yes (10, 50 μg/mL) |
No |
No |
N/s |
|
M7 |
Yes (10 μg/mL) |
Yes (100 μg/mL) |
Yes |
Decreased at 100 μg/mL |
|
M8 |
Yes (10 μg/mL) |
Yes (100 μg/mL) |
Yes |
Increased at 10 μg/mL, decreased at 100 μg/mL |
|
M9 |
Yes (10 μg/mL) |
Yes (100 μg/mL) |
Yes |
N/s |
|
N1 |
No |
No |
No |
N/s |
|
N2 |
Yes (10 μg/mL) |
No |
No |
N/s |
|
N3 |
No |
Yes (50, 100 μg/mL) |
No |
Decreased at 10 μg/mL |
|
N4 |
No |
Yes (50, 100 μg/mL) |
No |
N/s |
|
N5 |
No |
No |
No |
N/s |
|
N6 |
No |
No |
No |
Decreased at 100 μg/mL |
|
N7 |
No |
No |
No |
Decreased at 50, 100 μg/mL |
|
N8 |
No |
No |
No |
Decreased at 100 μg/mL |
|
N9 |
Yes (10, 50 μg/mL) |
No |
No |
No |
|
N10 |
No |
No |
No |
No |
Figure 1. Relative fold change in cell proliferation of primary human iMFBs after 24 hours’ treatment with nine likely-to-be bioactive matrikines at 10, 50 and 100 μg/mL. All comparisons with vehicle/control (serum-free medium + DMSO 1.25%) that were statistically significant are indicated with red. **p<0.005
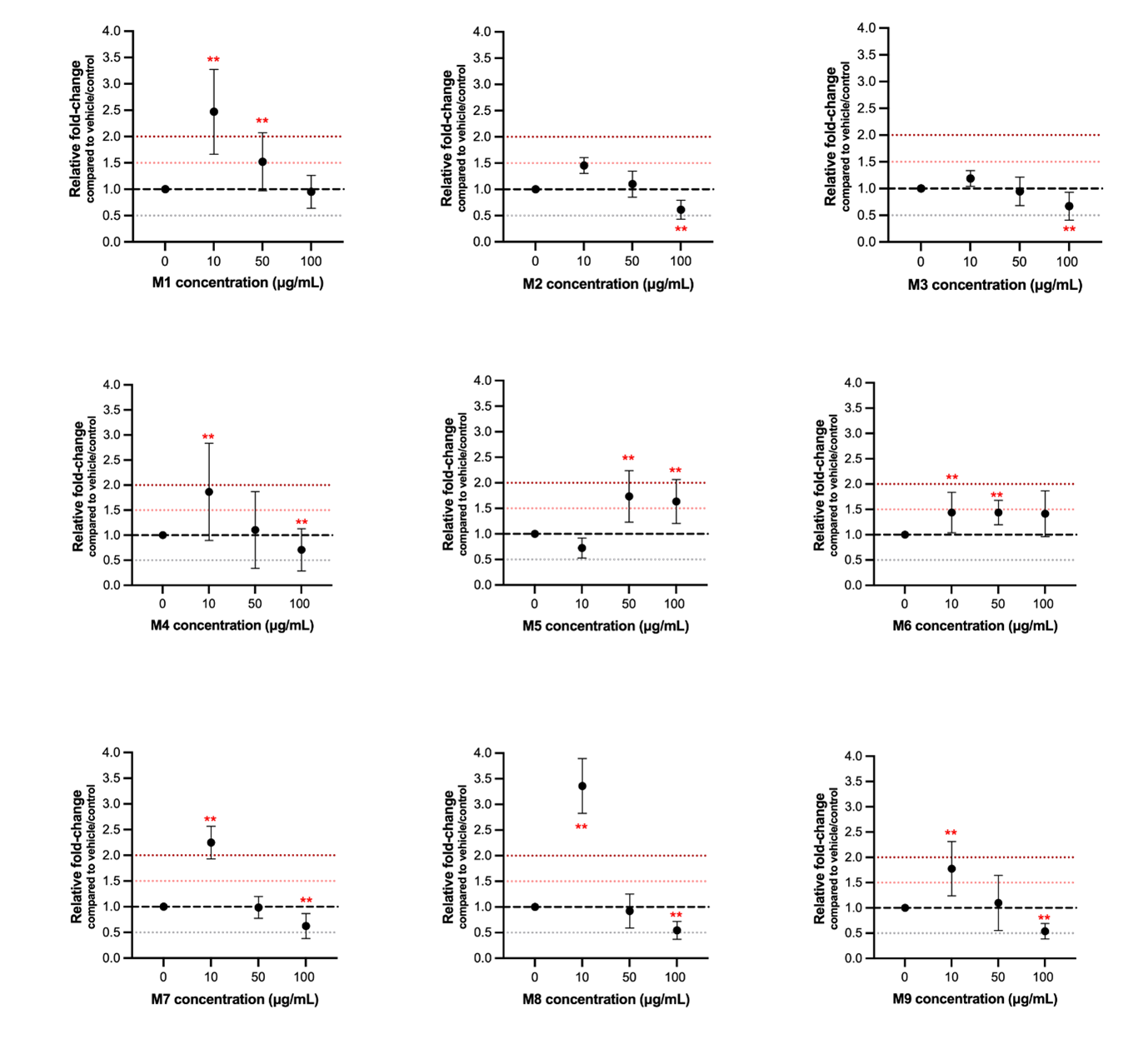
Figure 2. Quantitative polymerase chain reaction analysis for key markers of myofibroblasts. Fold change in comparison with vehicle/control (serum-free medium + DMSO 1.25%). N=3 biological replicates per condition. **p<0.005
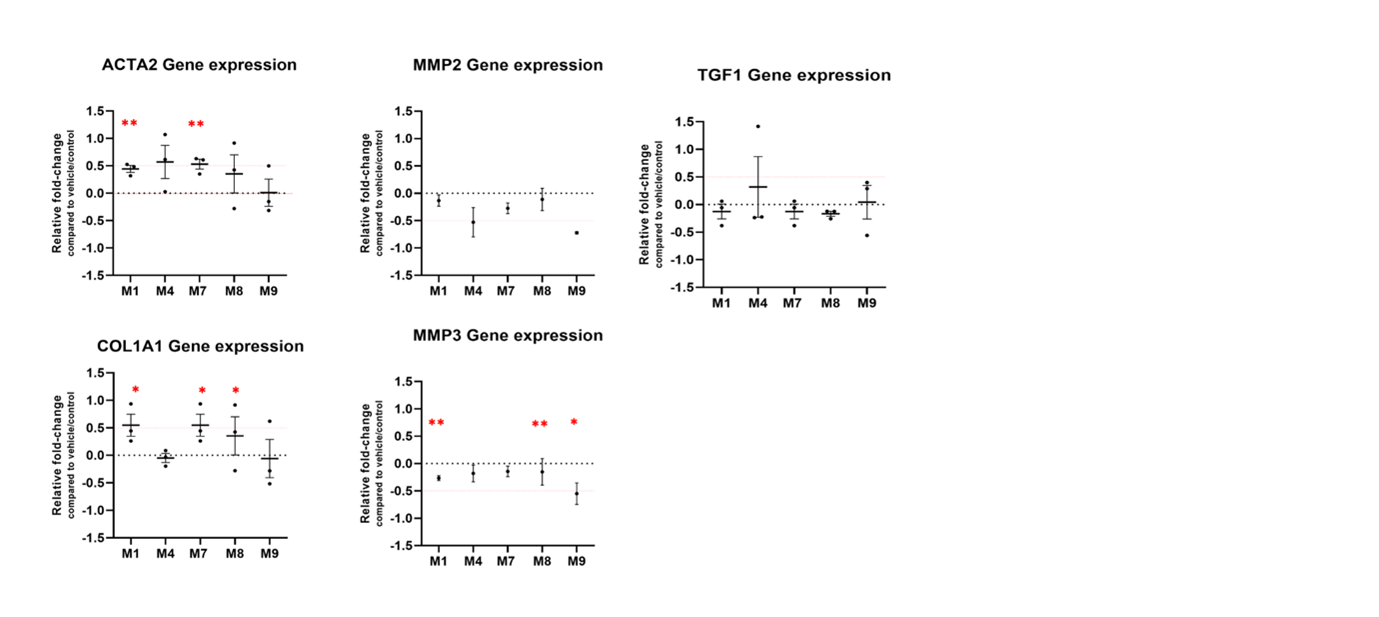
Figure 3. Enrichment plots for pathways related to pro-inflammatory and profibrotic mediators and VEGFR2-mediated vascular permeability that are only enriched in intestinal myofibroblasts when treated with selected matrikines in vitro. SFM: serum-free medium.