Report on the 10th SciCom Workshop at ECCO'22
Isabelle Cleynen, SciCom Member
 Isabelle Cleynen Isabelle Cleynen © ECCO |
This year we celebrated the 10th edition of the SciCom Workshop. What better way to do this than by looking ahead at future therapeutic IBD targets?
To set the scene, Marc Ferrante took us through the limitations of current IBD therapies, including the therapeutic ceiling, lack of information on niche indications (e.g. anal fistula, pouchitis), drugs that become available despite many remaining questions, and safety and economic aspects. However, there is a bright future ahead, with many opportunities to explore, such as the need for novel drugs with better efficacy, thinking beyond anti-inflammatory drugs, head-to-head trials, using drugs more efficiently, considering other aetiological factors, precision medicine and more.
Moving from this general overview, a series of talks presented specific possibilities for novel targets. First, Konrad Aden discussed whether the metabolome is the next wave to ride in IBD and postulated IBD as an immunometabolic disease. Konrad went into how we can use the understanding of the metabolome to modify established pharmacotherapy (e.g. microbial short-chain fatty acids as a predictor of therapy response), as well as how we can use it as a therapeutic target or agent itself (e.g. butyrate, tryptophan). While the extent to which metabolite alterations are the cause or the consequence of disease remains to be determined, the metabolome clearly represents an additional layer in intercellular and host–microbiome communication which we need to understand more deeply.
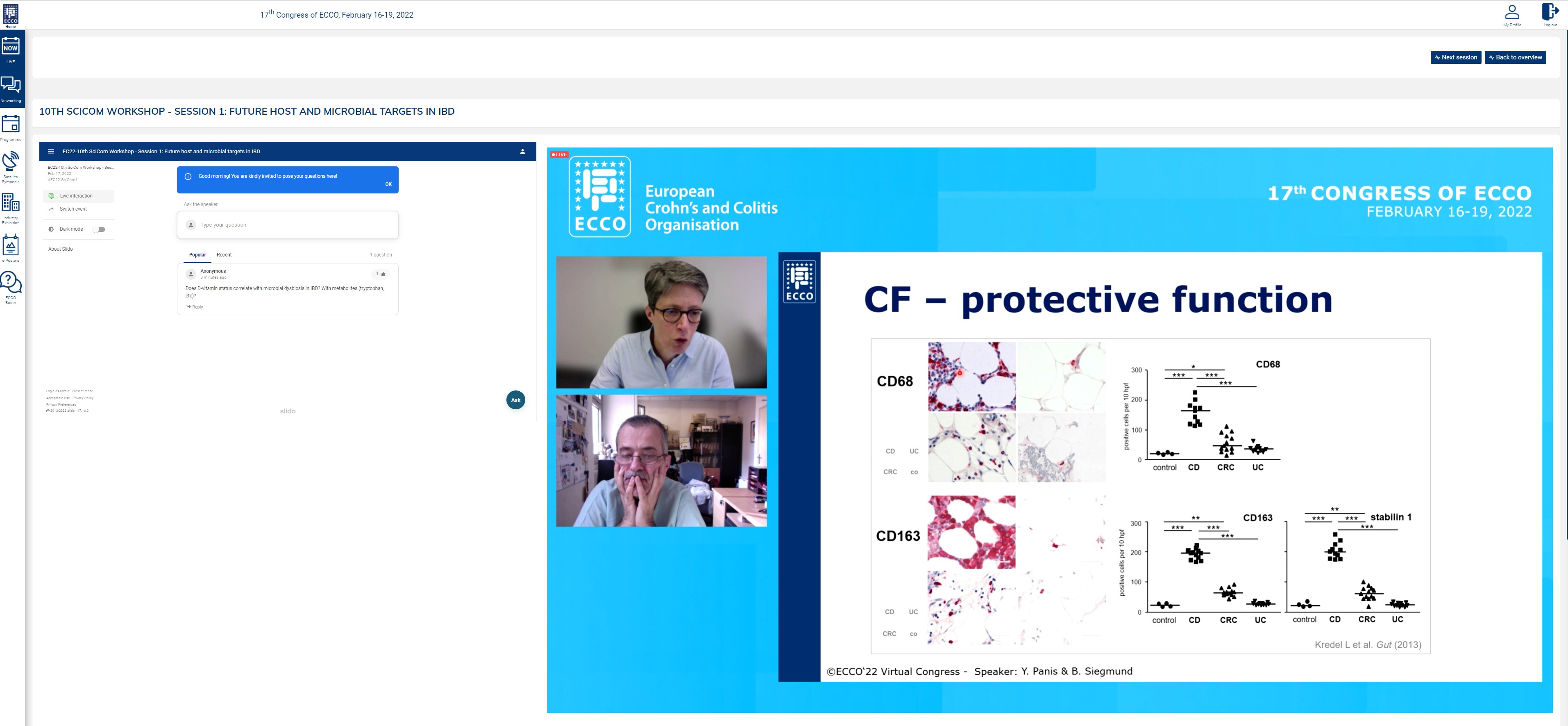
Next up was a brilliant tandem talk by Britta Siegmund and Yves Panis, in which they looked into the adipose tissue as a target of interest, both from a scientific and from a surgical perspective. This does not, however, seem an easy topic, as from both perspectives a protective as well as a disease-driving role of the mesentery has been seen. Britta presented work showing that on a cellular level, creeping fat builds an anti-inflammatory barrier to prevent bacteria from progressing; the surgeons also see examples where keeping the mesentery provides good long-term results. Conversely, other studies have shown fat tissue to have a pro-inflammatory role, and inflammatory cells have been observed in the mesorectum, suggesting it is best to remove it. Thus, there is no simple answer to whether creeping fat is protective or disease driving, as it depends on specific circumstances which we need to study further.
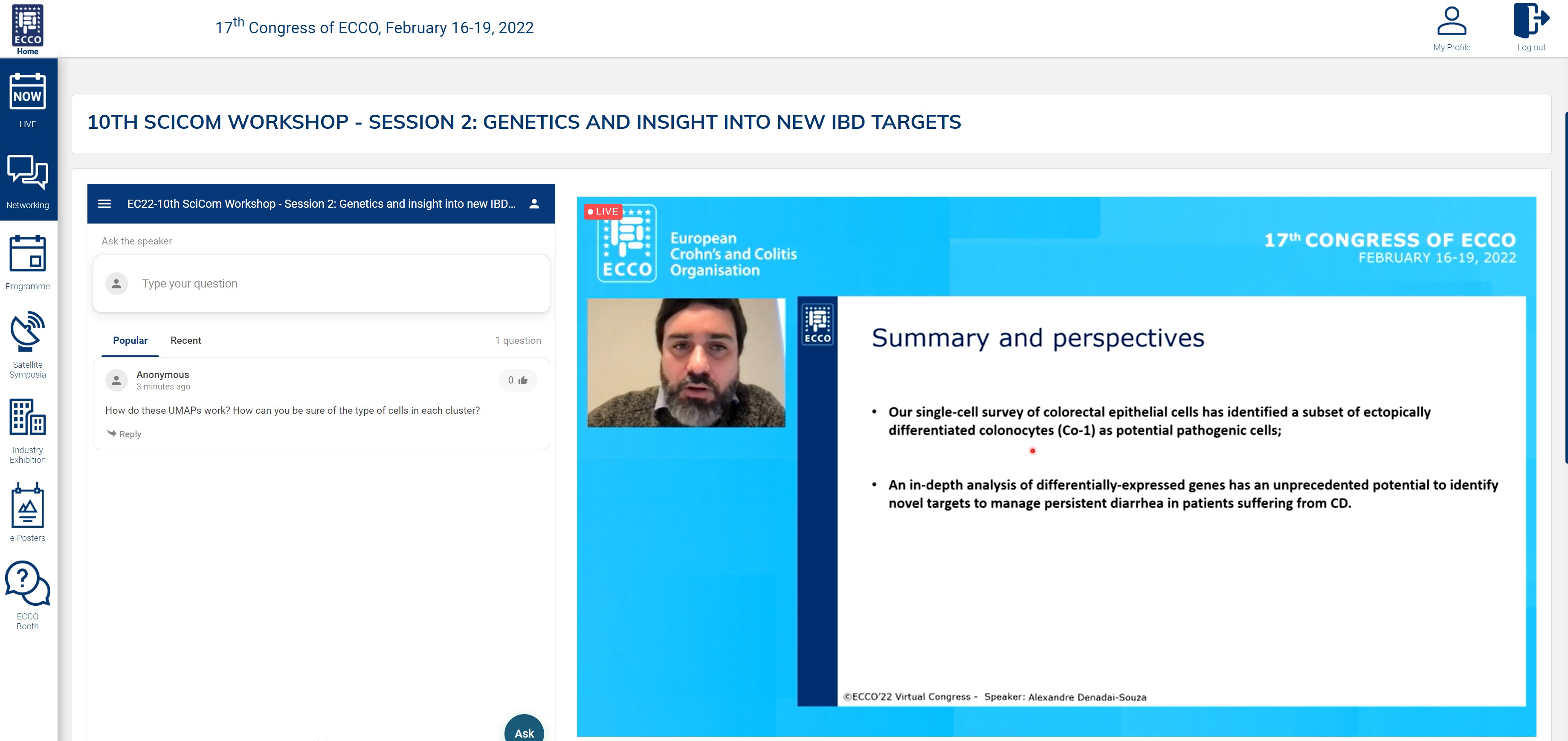
The second session started with insights from single cell sequencing studies. Alexandre Denadai Souza (kindly jumping in at the last minute) shared very elegant work exploring why some IBD patients in remission show persistent symptoms. Starting from crypts extracted from colonic mucosal biopsies, they found specific cellular perturbations in these patients, e.g. the presence of specific cell types like colonocytes type I. Only limited perturbations were seen in patients without symptoms. These studies will now be followed by in-depth analyses of differentially expressed genes to fully unfold the potential of single cell analysis to identify novel targets.
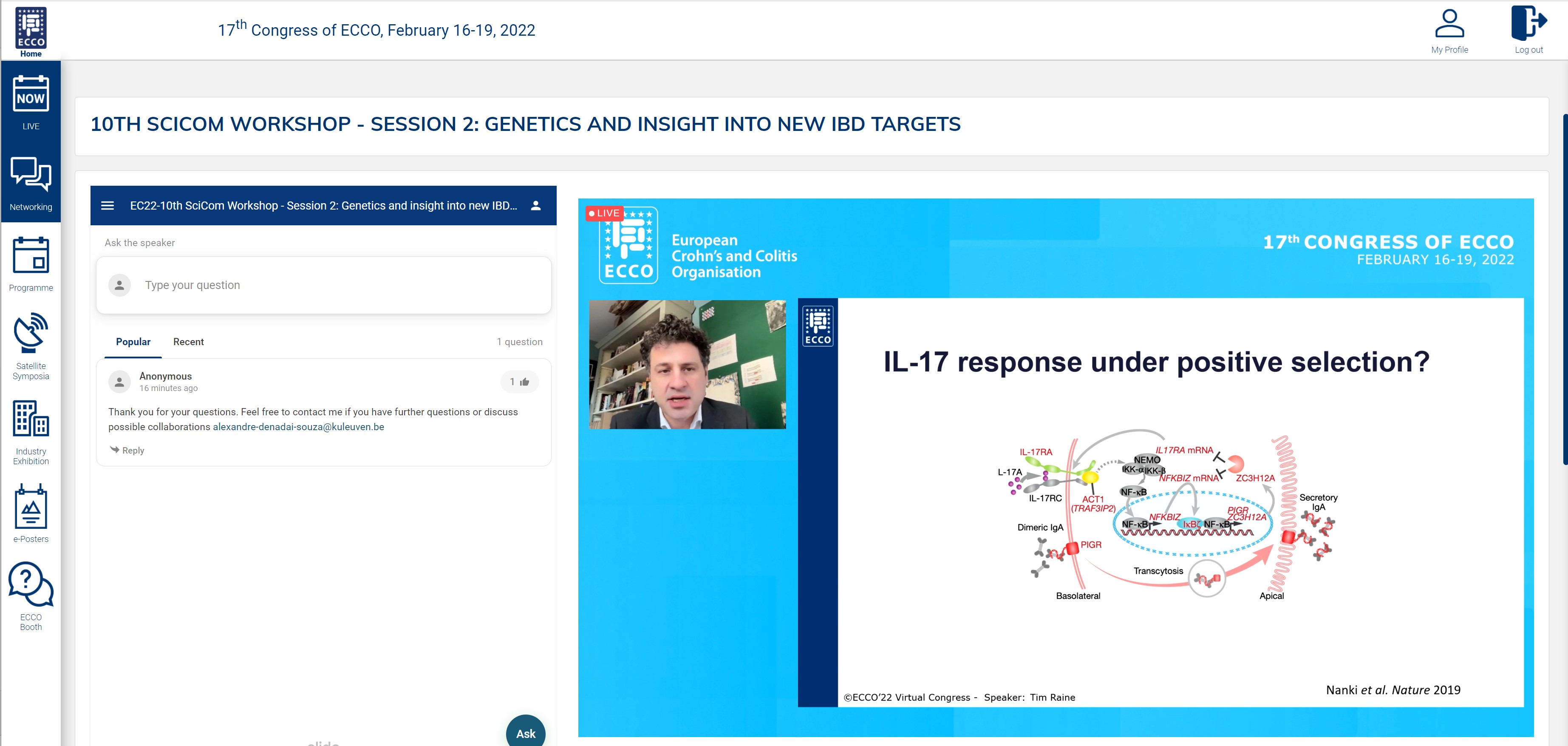
Then Tim Raine spoke on the role of somatic mutations in IBD. While the somatic mutation rate in uninflamed colon of patients was similar to that in healthy persons, Tim and his colleagues saw a threefold increase in the mutation rate in inflamed colon, as well as a different clonal structure, with stem cells escaping from the crypts and populating nearby crypts. The cause of these mutations mostly seems to be an accelerated normal ageing process. Furthermore, a high ratio of non-synonymous versus synonymous mutations in certain genes and pathways was seen, and positive selection of stem cells with specific mutations, e.g. in IL17 signaling genes. These mutant clones are then able to propagate over large areas of colonic mucosa. As to the question of what comes first – inflammation or somatic mutations – Tim believes it can probably be either.
Finally, Johan Van Limbergen discussed whether stem cell transplantation or gene editing could cure IBD. The final answer was clear: “No, except in some specific cases of monogenic IBD”. Johan discussed several reasons for this conclusion, taking NOD2 as an example: first, not everyone with NOD2 mutations will develop disease; second, there are effects beyond the gene itself, as NOD2 interacts with both immune genes and other processes like autophagy and ER stress; third, there may be whole gene effects as opposed to targeting of a single mutation. NOD2 is widely expressed, so problems might also arise in other tissues. Gene editing has also experienced many setbacks: mostly gene editing is done ex vivo, scaling up is difficult, there are off-target effects, etc.
While clearly a lot of work still lies ahead, this session highlighted some promising novel therapeutic intervention points for IBD, and avenues towards identifying them. We thank all the speakers for their exciting presentations and all attendees for posting many questions and much feedback in the chat space. All of you contributed to a very interesting and successful 10th SciCom Workshop. See you next year for the 11th edition.
Browse through the gallery:
Pictures are subject to copyright © ECCO