News from BIOCYCLE
Edouard Louis
 Edouard Louis © Edouard Louis |
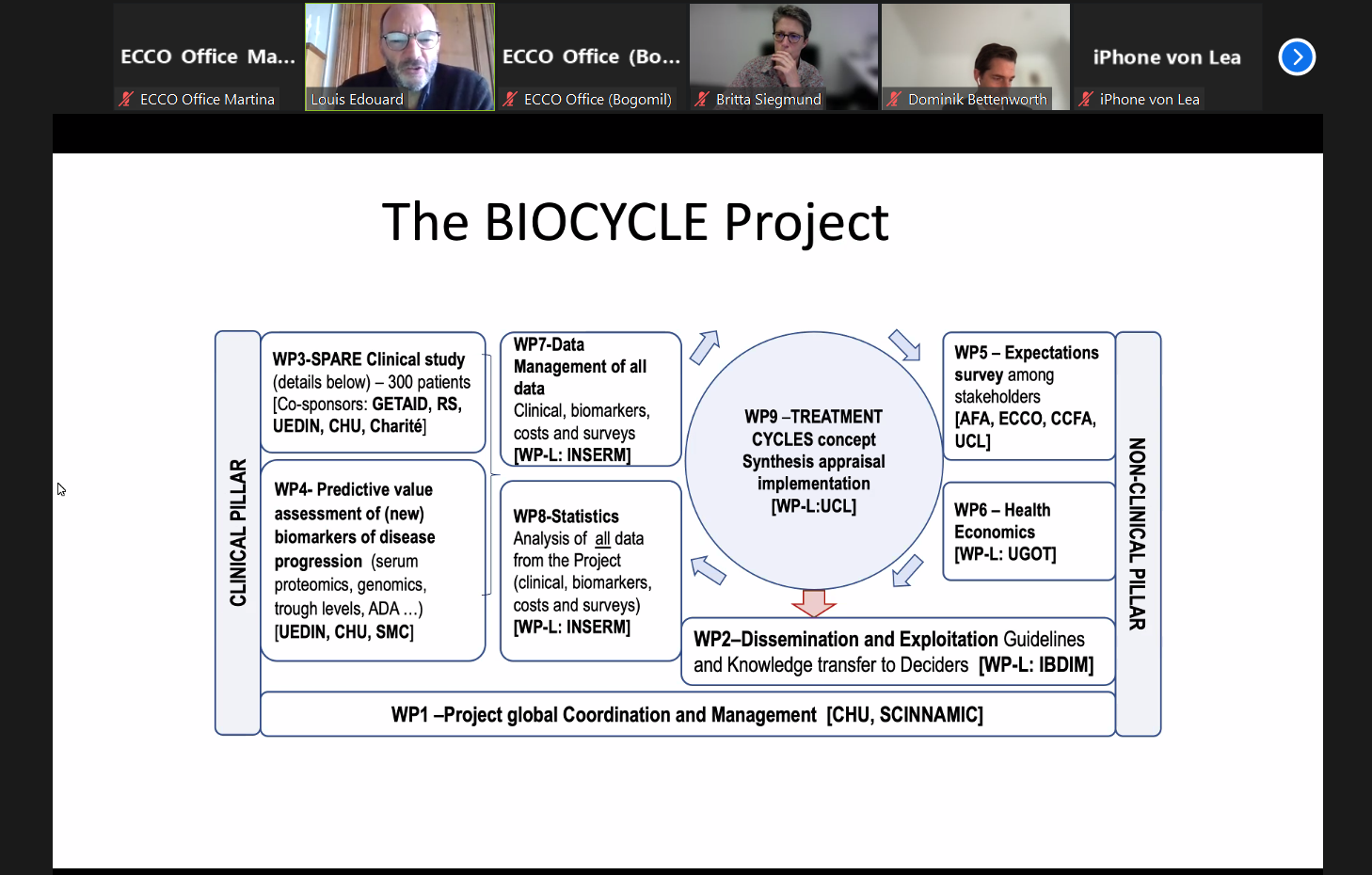
The BIOCYCLE project has now been ongoing for more than 7 years and is approaching its end. This project, funded by the European Commission under the Horizon 2020 programme, aims to explore different aspects of the difficult issue of treatment de-escalation in moderate to severe Crohn’s Disease first requiring a combination therapy with anti-TNF and antimetabolites to control the disease. Once the disease has been stabilised, an unsolved question is whether it is possible to de-escalate therapy. This question is important for several reasons, including safety, tolerance, quality of life and costs, to name the most prominent. BIOCYCLE includes a randomised three-arm, controlled clinical trial called SPARE, conducted on 200 patients in six European countries (France, United Kingdom, Belgium, Sweden, the Netherlands and Germany) and Australia, several patients’ and health care providers’ surveys in Europe and the United States, a biomarker research programme and pharmaco-economic analysis. ECCO is mainly involved in the monitoring of the project (through IBDIM) and is the work package leader for dissemination of the results. BIOCYCLE is a 7.5-year-long project and was launched in April 2015.
The global aim of BIOCYCLE is to try and bring an integrated, multidimensional and tailored response to the issue of treatment de-escalation in Crohn’s Disease, also taking into account patients’ preferences and perspectives.
What has been achieved so far?
All work packages have now made huge progress and several are closed or close to the end. The amount of data generated is impressive and will feed further research in the field.
The SPARE clinical trial has been completed and the primary and main secondary endpoints have been analysed. Oral presentations were made at ECCO and DDW 2022. The first clinical paper has just been accepted for publication in Lancet Gastroenterology and Hepatology and will be online soon. The main finding of this trial is that there is an increased risk of relapse and loss of time in remission in patients on combination therapy who stop infliximab, compared with those who continue infliximab in the context of either monotherapy or combination therapy. The trial also shows that immunosuppressant withdrawal is not associated with an increased risk of relapse or of drug immunogenicity. The results help to define risk factors for relapse and for failure of therapy if patients are de-escalated from combination therapy and demonstrate that the majority of patients experiencing relapse on infliximab withdrawal rapidly respond to re-treatment and maintain remission over two years. While the trial demonstrates that withdrawal of infliximab is associated with an increased risk of relapse, it also shows that the effects of relapse can be effectively and rapidly treated in most patients. Treatment decisions should be tailored to each patient’s circumstances in order to optimise the balance of potential benefit and harm.
The health care providers’ and patients’ surveys, coordinated by the CCFA in the United States and by the patients’ association, AFA, in France, with the help of ECCO, in which more than 400 patients and close to 300 doctors participated, were completed several years ago and the results were presented at ECCO Congress in 2017 and 2018. The manuscript summarising the most important results has been fully published in Clinical Gastroenterology and Hepatology.
The biological samples collected at baseline and at different time points during follow-up have started to generate exciting data through a collaboration involving Oxford, Liège and Tel Aviv. Different proteomic approaches (including mass spectrometry-based techniques and proximity extension assay) have disclosed a series of potential predictors of short- and longer-term relapse after treatment withdrawal. These results actually confirm and validate previous results generated on the similar STORI cohort (GETAID-2004-2009) comprising patients stopping infliximab. Short-term relapsers are characterised by an increase in a series of acute phase reactants and proteins associated with ongoing inflammation, while longer term relapsers seem to harbour a more heterogeneous profile. Data have also been generated in the fields of metabolomics, glycomics, DNA methylation and pharmacokinetics. These data are currently being individually analysed and will be further merged for a multi-omics approach.
The pharmaco-economic work package led by Gothenburg University has started and a theoretical pharmaco-economic model for the cycling of immunomodulators or biologics has been generated based on a Markov-type decision tree model, which was fully published in JCC in 2019. The final analysis of this model in the context and with the data of the SPARE trial is currently being performed by our colleagues at Gothenburg University. A dedicated study of the evolution of health-related quality of life is also ongoing, with our Swedish colleagues, based on the material generated by the SPARE trial.
The final aim of BIOCYCLE is to generate knowledge and processes to help decision-making regarding treatment withdrawal and cycles in Crohn’s Disease and, more broadly, in immune-mediated inflammatory diseases. To this end, ECCO-IBDIM has organised a Topical Review on treatment cycles in CD. An expert panel was convened to review the published literature and agree a series of consensus practice points. The expert consensus aimed to provide evidence-based guidance to support shared decision-making with the patient. The consensus evaluated multiple aspects of biological treatment discontinuation and cycling, including the risk of relapse after elective treatment discontinuation, predictors of likely relapse or remission, safety, patient preferences and pharmaco-economic aspects. Crucially, it was concluded that discussions about biological treatment discontinuation and cycling should be individualised, to enable shared decision-making by patients and clinicians. The final manuscript of this Topical Review will be submitted to JCC by the end of December 2022. In parallel to this process, our colleagues from the public health school of UC Louvain are developing a pathway of care for patients with Crohn’s Disease who are contemplating treatment de-escalation. This work, which has also included a broad literature review and focus group discussions, is also in its final phase.
|
|
One further important aspect of the BIOCYCLE project is dissemination of the concept and of the results generated by the action. To this end, ECCO-IBDIM has organised three BIOCYCLE dissemination meetings. The first was held in September in Gothenburg, the second was held virtually from Berlin in October and the third will be held virtually from Nice at the end of November 2022. The content of these dissemination meetings is based on the Topical Review highlighted above and illustrated through exemplative and interactive clinical cases.
The last administrative and scientific report will be prepared in January 2023, immediately after the official end of the grant on the December 31, 2022, and will be submitted to the European Commission in the following weeks. Most of the initially foreseen deliverables will have been achieved at that date and the material generated will continue to feed exciting research in the field of treatment de-escalation over the coming years.
For more information, see the BIOCYCLE website: http://biocycle-project.eu/; EU reference: grant agreement No 633168 – BIOCYCLE (PHC-13-2014)
Browse through the gallery:
Pictures are subject to copyright © ECCO