DOP10 Intestinal ultrasound response and transmural healing after ustekinumab induction in Crohn’s disease: Week 16 interim analysis of the STARDUST trial substudy
T. Kucharzik1, R. Wilkens2,3, G. Maconi4, M.A. D’Agostino5, M. Le Bars6, M. Nazar7, S. Sloan8, M. Lahaye9, L. Ni10, E. Ercole11, M. Alloca12, N. Machková13, C. Maaser14
1Klinik für Allgemeine Innere Medizin und Gastroenterologie, Klinikum Lüneburg, Lüneburg, Germany, 2Gastro Unit Section of Medicine, Hvidovre Hospital, Copenhagen, Denmark, 3Digestive Disease Center, Bispebjerg Hospital, Copenhagen, Denmark, 4Gastroenterology Unit, Department of Biomedical and Clinical Sciences, ‘Luigi Sacco’ University Hospital University of Milan, Milan, Italy, 5Ambroise Paré Hospital APHP-Paris Saclay, University INSERM U1173 Laboratoire d’Excellence INFLAMEX, Rheumatology Department, Paris, France, 6Department of Medical Affairs, Janssen-Cilag SARL, Issy-les-Moulineaux, France, 7Department of Medical Affairs, Janssen-Cilag Polska Sp. z o.o, Warsaw, Poland, 8Department of Medical Affairs, Janssen Scientific Affairs LLC, Horsham, PA, USA, 9Department of Medical Affairs, Janssen-Cilag B.V., Breda, The Netherlands, 10Department of Medical Affairs, Janssen-Cilag, Moscow, Russian Federation, 11Gastroenterology Unit, Mauriziano Hospital, Turin, Italy, 12Department of Gastroenterology, Humanitas University, Milan, Italy, 13Clinical Center ISCARE, Clinical and Research Center for Inflammatory Bowel Diseases, Prague, Czech Republic, 14Ambulanzzentrum Gastroenterologie, Klinikum Lüneburg, Lüneburg, Germany
Background
Intestinal ultrasound (IUS) is a non-invasive tool for evaluating transmural disease activity in Crohn’s disease (CD) patients. We report interim Week (W)16 results from an IUS substudy of STARDUST, a phase 3B randomised trial of CD patients treated with ustekinumab (UST), comparing a treat-to-target (T2T) maintenance treatment strategy vs. standard of care (SoC). The aim of the substudy was to assess changes in IUS parameters, including transmural response to UST induction therapy.
Methods
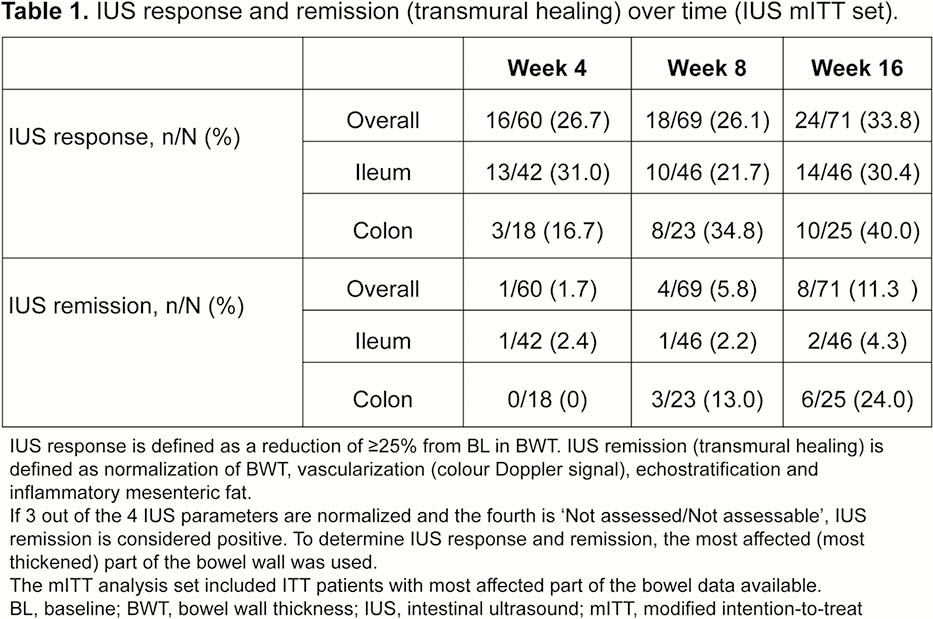
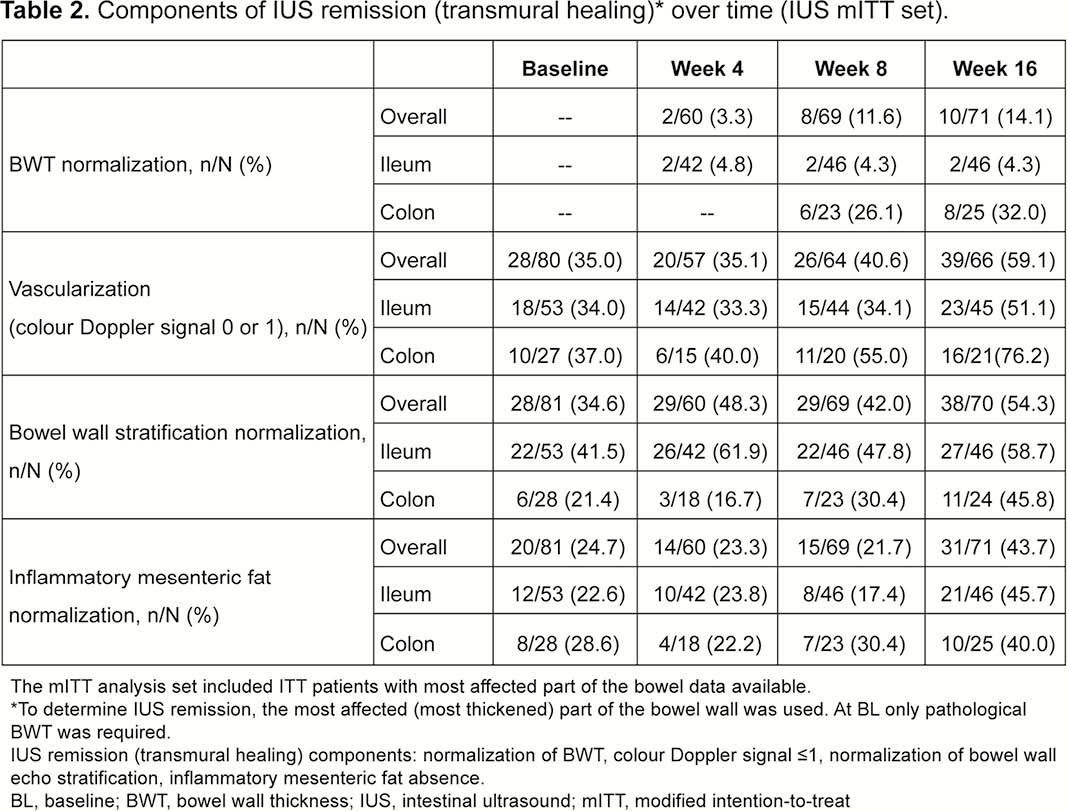
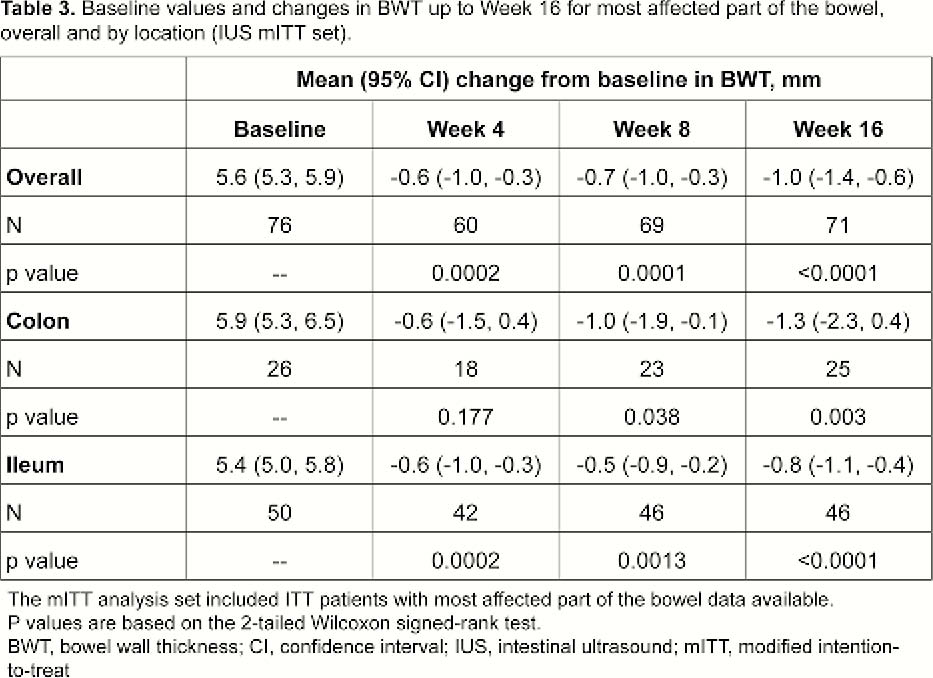
Patients (≥18 years) with moderate–severe active CD (CD Activity Index [CDAI] 220–450 and simple endoscopic index for CD [SES-CD] ≥3) who failed conventional therapy and/or 1 biologic, received weight-based IV UST dosing of ~6mg/kg at Week 0, then SC UST 90mg at W8. At Week 16, patients with CDAI reduction ≥70 points were randomised (1:1) to T2T or SoC treatment arms. Exclusion criteria: characteristics precluding IUS visualisation of affected bowel segments and normal bowel wall thickness (BWT) (≤2.0mm terminal ileum; ≤3.0mm colon) for all segments at baseline (BL). Key IUS endpoints assessed at W4, W8 and W16 (central reading): IUS response ≥25% BWT reduction from BL; BWT change from BL (mm); IUS remission (transmural healing) ‒ BWT normalisation, colour Doppler signal ≤1, normal echo stratification, and absence of inflammatory fat. The most affected bowel segment at BL was used for all IUS parameters. Correlations/% agreement between IUS response/remission and clinical (CDAI70, clinical response/remission), biomarker (CRP/FCal levels) and endoscopy outcomes (SES-CD scores) were assessed.
Results
The analysis included 82/94 patients enrolled in the IUS substudy;
Conclusion
This was the first international, interventional, multicentre study using IUS in CD. IUS response to UST was detected as early as Week 4, and a clinically meaningful % of patients achieved transmural healing, primarily in the colon, at W16. IUS could be a valuable tool to detect early response to treatment in CD. Future studies need to confirm whether early IUS response is predictive of long-term outcomes for CD patients.


