DOP12 Efficacy of ustekinumab for ulcerative colitis through 2 years: Results of the UNIFI maintenance study and long-term extension
R. Panaccione1, W.J. Sandborn2, B.E. Sands3, C. Marano4, C.D. O’Brien4, H. Zhang5, J. Johanns5, Y. Zhou5, L. Peyrin-Biroulet6, T. Hisamatsu7, S. Danese8, UNIFI Investigators
1University of Calgary, Inflammatory Bowel Disease Center, Calgary, Canada, 2University of California San Diego, Gastroenterology, La Jolla, USA, 3Icahn School of Medicine at Mount Sinai, Gastroenterology, New York, USA, 4Janssen Research and Development- LLC, Immunology, Spring House, USA, 5Janssen Research and Development- LLC, Clinical Biostats, Spring House, USA, 6Nancy University Hospital, Gastroenterology, Nancy, France, 7Kyorin University School of Medicine, Internal Medicine, Tokyo, Japan, 8Humanitas Research Hospital, Gastroenterology, Milan, Italy
Background
Ustekinumab (UST) is a mAb to IL-12/23p40 that is approved for moderate-to-severe ulcerative colitis (UC). The UNIFI maintenance study evaluated SC UST through 1 year in patients who responded to UST IV induction. Patients who completed the maintenance study could enter a long-term extension (LTE) through 220weeks. Here, we report the efficacy of UST through 92weeks for patients randomised in the maintenance study.
Methods
Patients who were in clinical response 8weeks after UST induction were randomised to SC PBO (
Results
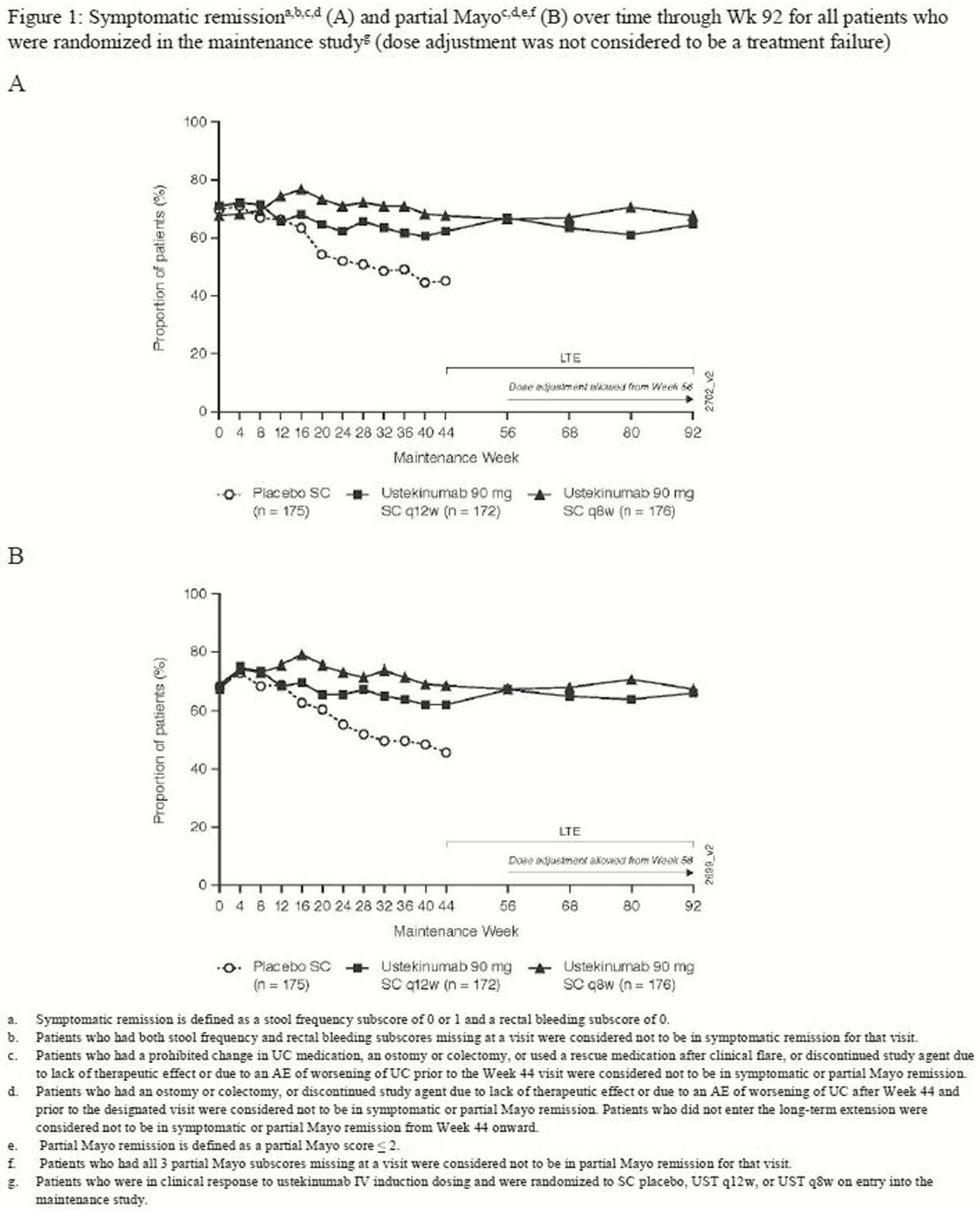
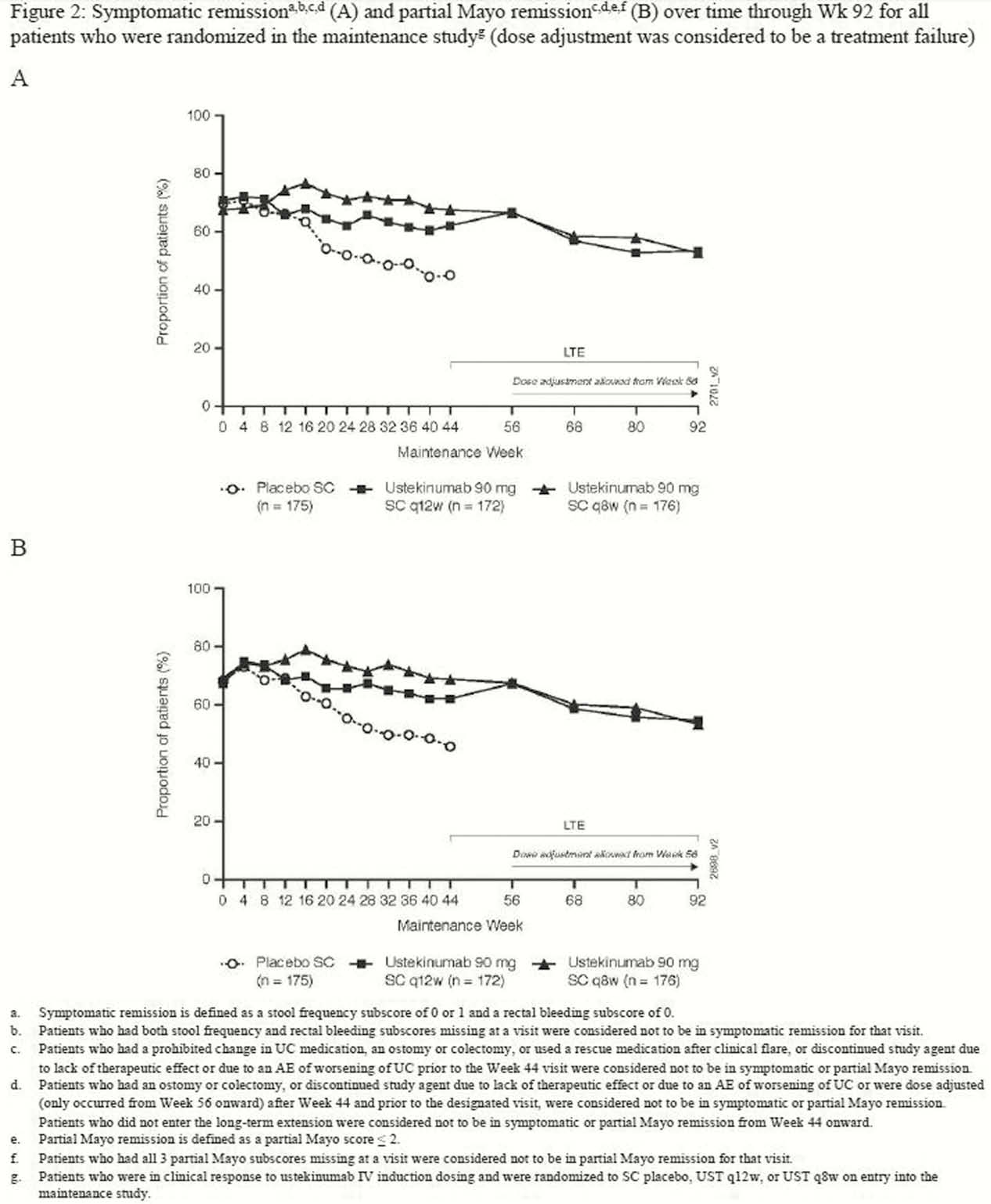
Symptomatic and partial Mayo remission were sustained through Week 92, with no clinically meaningful differences between the q12w and q8w dose groups (Figure 1 and 2). When dose adjustment was considered to be part of the treatment experience (ie, not a treatment failure), 66.1 % and 67.0% of patients randomised to q12w and q8w, respectively, were in symptomatic and partial Mayo remission at Week 92 (Figure 1). When dose adjustment was considered to be a treatment failure in the analysis, 53.2% and 54.0% of patients were in symptomatic and partial Mayo remission, respectively (Figure 2). The safety profile of UST through Week 96 was consistent with that previously reported for 1 year.
Conclusion
The efficacy of UST in patients with moderate-to-severe UC has maintained through 92weeks with q12w or q8w SC dosing when dose adjustment was considered to be part of the treatment experience.


