DOP17 Identification of biomarkers and mechanistic insight for upadacitinib in ulcerative colitis: Analysis of serum inflammatory mediators in the phase 2b U-ACHIEVE study
S. Vermeire1, H. Guay2, B. Verstockt3, J. Fann4, J. Cheng4, F. Hong4, W.J. Lee5, A. Lacerda6, W. Zhou6, S. Danese7
1Department of Translational Research Centre for GastroIntestinal Disorders TARGID, KU Leuven, Leuven, Belgium, 2Immunology Clinical Development, AbbVie Inc., Worcester, USA, 3Department of Chronic Diseases, Metabolism and Ageing, CHROMETA, KU Leuven, Leuven, Belgium, 4Discovery and Exploratory Statistics, AbbVie Inc., Worcester, USA, 5Health Economics and Outcomes Research HEOR, AbbVie Inc., Worcester, USA, 6Global Pharmaceutical Research and Development, AbbVie Inc., North Chicago, USA, 7Department of Gastroenterology, Instituto Clinico Humanitas, Milan, Italy
Background
The U-ACHIEVE trial evaluated upadacitinib (UPA), an oral JAK1 selective inhibitor, in patients with moderately to severely active ulcerative colitis (UC). Patient-reported and endoscopic outcomes improved after UPA treatment. This analysis used pharmacodynamic profiling to link changes in serum biomarkers to changes in UC disease activity, and to assess the UPA mechanism of action in UC.
Methods
U-ACHIEVE (NCT02819635) was a randomised, double-blind, placebo (PBO)-controlled phase 2b clinical trial. Adults with an inadequate response, loss of response, or intolerance to corticosteroids, immunosuppressants, or biologic therapies were randomised to receive 7.5, 15, 30, or 45 mg UPA once daily or PBO for 8 weeks (weeks). Serum samples (baseline [BL], weeks 2, 4, and 8) were analysed by OLINK® inflammation panel (92 proteins) and by Singulex immunoassay for interleukin-1b (IL-1b), IL-17A, IL-17F, and IL-22. Protein-level changes were analysed by a mixed-effect model; BL protein level was adjusted as a covariate; treatment group, time point, and their interaction were included as fixed effects. Spearman rank-correlation coefficients were used to determine the relationship between changes of serum biomarker levels and improvements in adapted Mayo scores and endoscopic subscores. Multiplicity adjusted
Results
Paired BL and week 8 serum samples were available from 114 patients (PBO,
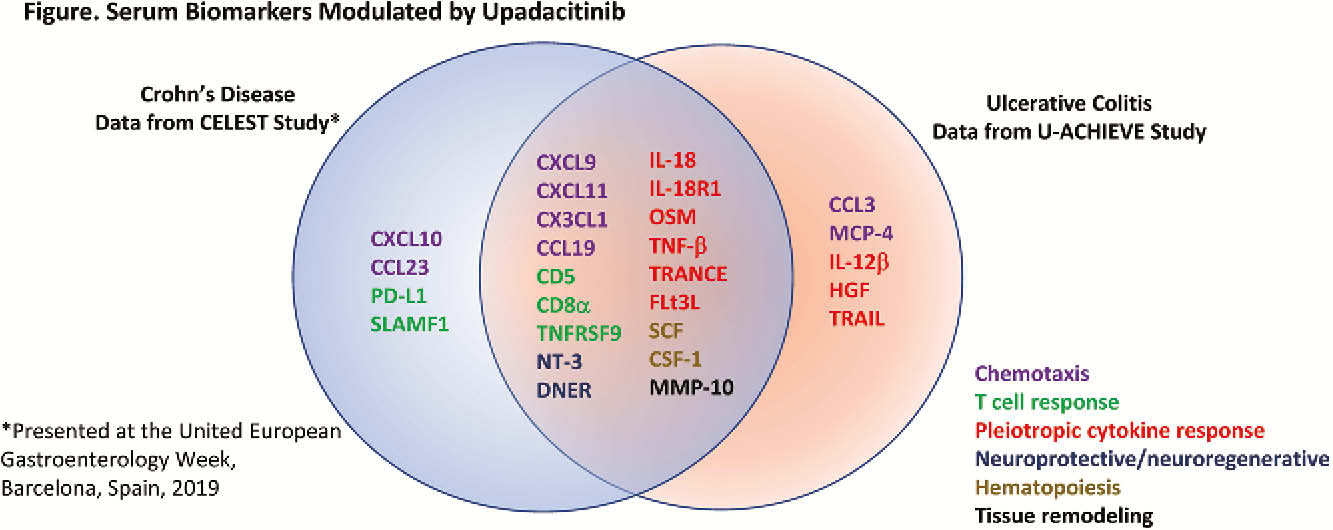
Conclusion
UPA modulated expression of serum pro-inflammatory mediators found in pathways associated with the pathogenesis of UC, including immune cell migration, type I/II IFN responses, T-cell responses, macrophage and dendritic cell activity, haematopoiesis, neuroprotection, and mucosal repair. Consistent correlations were observed between changes in biomarker expression and improvements in disease activity and symptoms of UC.


