DOP21 Time to first treatment with biologic agents for ulcerative colitis and Crohn’s disease across four Nordic countries: Results from the TRINordic study
M. Høivik1, M. Lördal2, J. Burisch3, E. Langholz4, T. Knudsen5, M. Voutilainen6, B. Moum1, K. Anisdahl1, B. Saebo7, P. Haiko8, C. Malmgren9, M. Coskun10, H.O. Melberg11
1Department of Gastroenterology, Oslo University Hospital, Oslo, Norway, 2Department of Gastroenterology and Hepatology, Danderyds Hospital, Stockholm, Sweden, 3Department of Gastrounit- Medical Division, Hvidovre University Hospital, Hvidovre, Denmark, 4Department of Medical Gastroenterology, Herlev Hospital, Herlev, Denmark, 5Department of Medicine, Hospital South West Denmark, Department of Regional Health Research, University of Southern Denmark, Odense, Denmark, 6Department of Gastroenterology, University of Turku, Turku, Finland, 7Takeda AS, Medical Affairs, Asker, Norway, 8Takeda Oy, Medical Affairs, Helsinki, Finland, 9Department of Medical Affairs, Takeda Pharma AB, Stockholm, Sweden, 10Department of Medical Affairs, Taastrup, Denmark, 11Department of Health Management and Health Economics, Institute of Health and Society, University of Oslo, Oslo, Norway
Background
Real-world data on time from diagnosis to first biologic treatment is limited for ulcerative colitis (UC) and Crohn’s disease (CD) patient populations.
Methods
This retrospective observational study collected data from the National Patient Registries and National Prescription Registries in Sweden (data on biologic use was only available for Stockholm [STK], Norway [NOR], Denmark [DEN]) and one university hospital database (Turku, Finland [FIN]) during 2010–2017 to investigate time from diagnosis to first biologic treatment for UC and CD. Patients with ≥2 ICD-10 diagnosis codes for UC (K51) or CD (K50) from 2010 or later were included; patients were classified according to their last code. The look-back period for SWE was until 2000, for NOR until 2008, for DEN until 1995, and for FIN until 2004. Time to first biologic was defined as the period from the first UC or CD code to first biologic record. In FIN, it was only possible to investigate infliximab (IFX).
Results
A total of 47,568 patients were included (STK
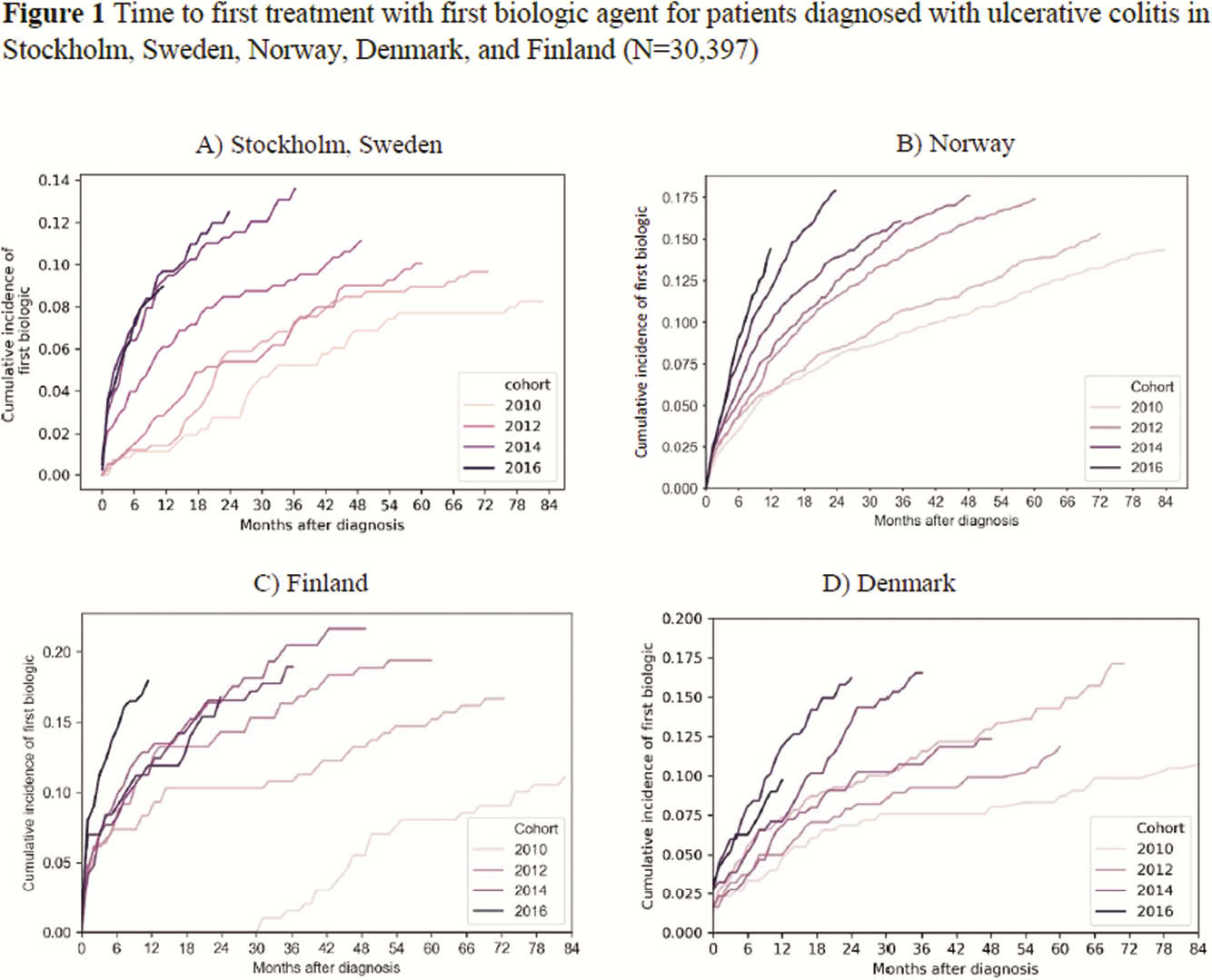
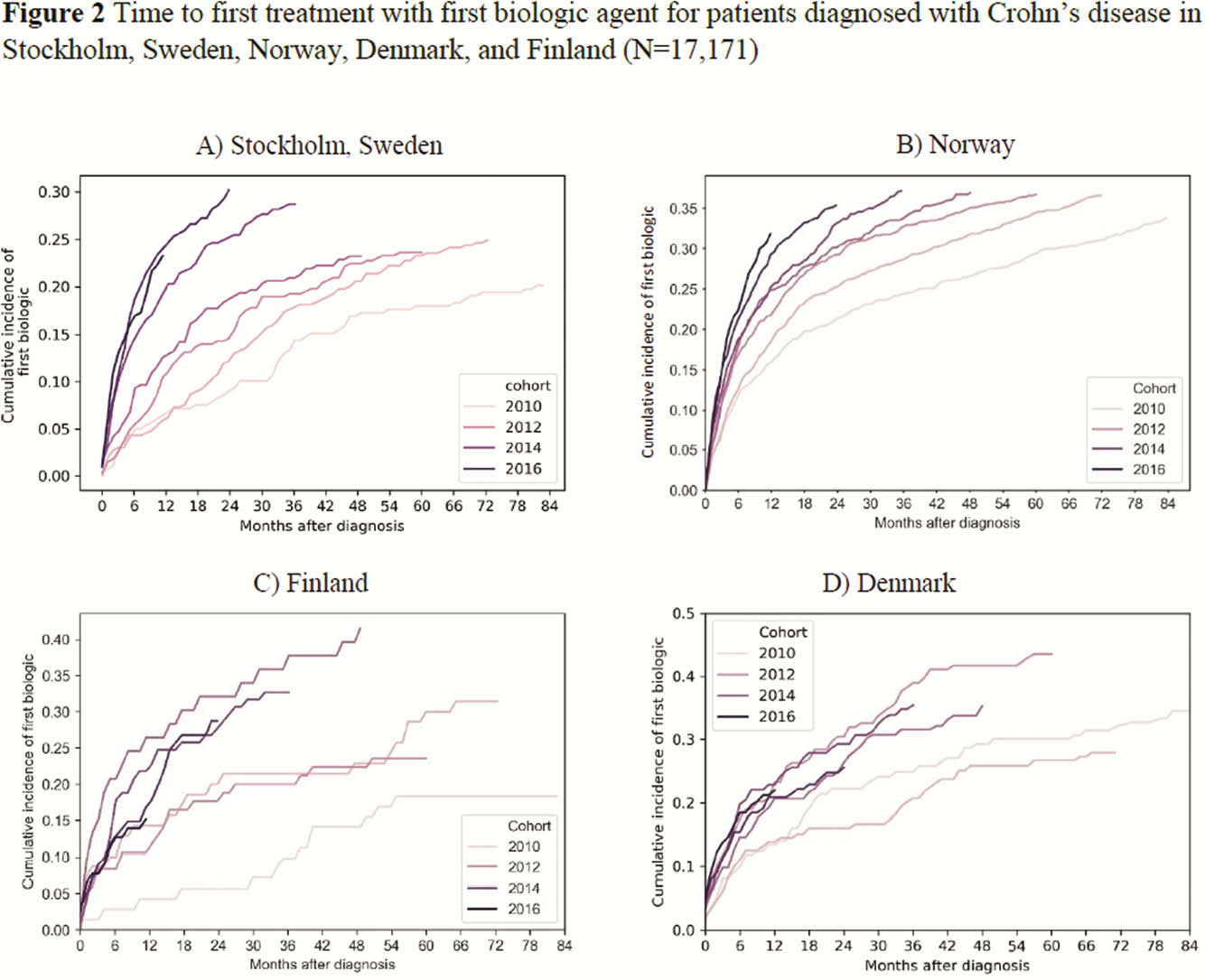
Conclusion
This retrospective observational study of >45 000 patients with inflammatory bowel disease in four Nordic countries showed reduced time between diagnosis and first biologic from 2010 to 2017, with the shortest time between diagnosis and first biologic in Norway. IFX was most commonly used.


