DOP24 Patient-reported health-related quality-of-life outcomes with vedolizumab vs. adalimumab treatment of ulcerative colitis: Results of the VARSITY trial
E.V. Loftus Jr1, S.W. Schreiber2, S. Danese3, L. Peyrin-Biroulet4, J.F. Colombel5, B.E. Sands5, S. Wang6, J. Chen7, R.A. Lirio8
1Division of Gastroenterology and Hepatology, Mayo Clinic College of Medicine, Rochester, USA, 2Department of Medicine, University Hospital Schleswig-Holstein, Kiel, Germany, 3Department of Gastrointestinal Immunopathology, Humanitas University, Milan, Italy, 4Department of Gastroenterology, Nancy University Hospital, Nancy, France, 5Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, USA, 6Global Outcomes Research and Epidemiology, Takeda, Cambridge, USA, 7Statistics and Quantitative Sciences, Takeda, Cambridge, USA, 8Clinical Science, Takeda, Cambridge, USA
Background
Patients with ulcerative colitis (UC) experience substantial impairment in quality of life (QOL). Patient QOL endpoints are important measures of treatment outcome. We evaluated the effects of intravenous vedolizumab vs. adalimumab on QOL in VARSITY, the first head-to-head trial comparing the efficacy and safety of biologics in patients with moderately to severely active UC.
Methods
VARSITY was a phase 3b, double-blind, double-dummy, randomised trial (NCT02497469; EudraCT 2015-000939-33). QOL was assessed using the inflammatory bowel disease questionnaire (IBDQ) at baseline, Week (Week) 30, and Week 52. Endpoints included clinically meaningful IBDQ improvement (defined as an increase in total score of ≥16 points from baseline to Week 52), IBDQ remission (defined as a total score of >170 points at Week 52) and change from baseline in IBDQ-specific domain scores (bowel symptoms, systemic symptoms, emotional function, and social function) at Week 30 and Week 52. Serum C-reactive protein (CRP) and faecal calprotectin (FCP) were also assessed as indicators of disease activity.
Results
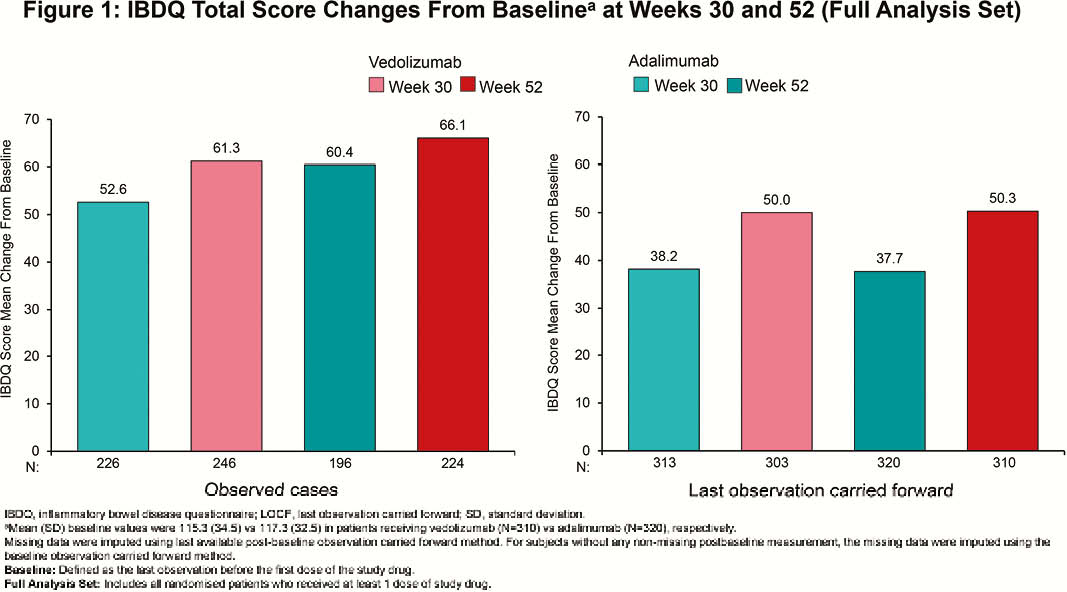
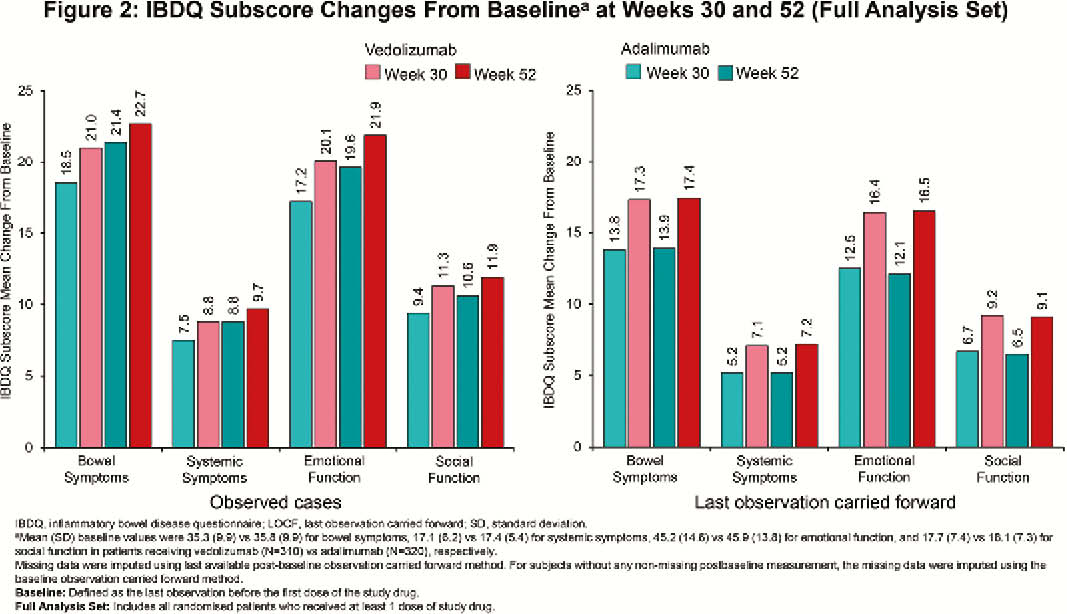
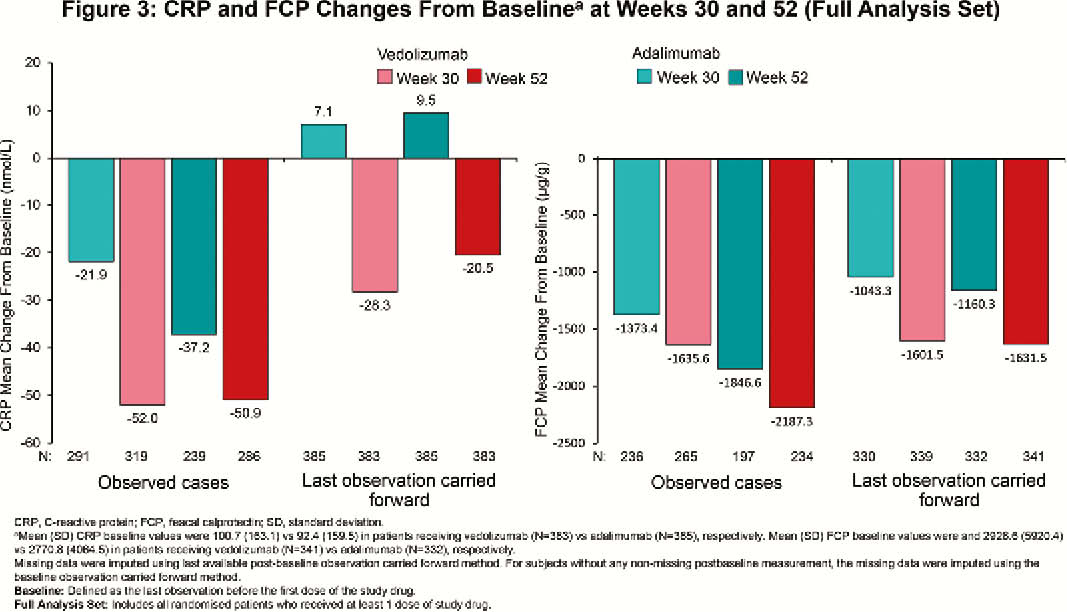
Among randomised patients, 383 (vedolizumab) and 386 (adalimumab) patients received ≥1 dose of study drug (N=769). At Week 52, clinically meaningful IBDQ improvement was observed in 52.0% (vedolizumab) vs. 42.2% (adalimumab) of patients (treatment difference 9.7%; 95% confidence interval [CI], 2.7% to 16.7%), while IBDQ remission was achieved by 50.1% (vedolizumab) vs. 40.4% (adalimumab) of patients (treatment difference 9.6%; 95% CI, 2.8% to 16.5%). Mean (standard deviation [SD]) changes in IBDQ total score from baseline for observed cases favoured vedolizumab over adalimumab (Week 30: 61.3 [39.8] vs. 52.6 [42.8]; Week 52: 66.1 [41.8] vs. 60.4 [42.2]; Figure 1). IBDQ subscores showed similar favourable trends for vedolizumab (Figure 2). At Week 52, mean (SD) changes from baseline in CRP for patients treated with vedolizumab vs. adalimumab were –50.9 (174.8) nmol/l vs. –37.2 (169.2) nmol/l and for FCP were –2187.3 (7440.4) µg/g vs. –1846.6 (4560.6) µg/g (Figure 3). Among patients with FCP >250 µg/g at baseline, the proportion of patients achieving FCP ≤250 µg/g was 33.9% vs. 24.5% at Week 30 and 35.2% vs. 28.9% at Week 52 for patients treated with vedolizumab vs. adalimumab, respectively.
Conclusion
Based on IBDQ total score and subscores, more patients with UC treated with vedolizumab than with adalimumab achieved clinically meaningful improvement and clinical remission. Reduced inflammation, as indicated by improvements in CRP and FCP, was consistent with improvements in QOL.


