DOP26 Real-life dosing patterns and concomitant drug use among ustekinumab-treated patients with Crohn’s disease
C.G. af Björkesten1, T. Ilus2, T. Hallinen3, E. Soini3, A. Eberl1, K. Hakala4, M. Heikura5, E. Hirsi6, A. Jussila2, M. Kellokumpu7, R. Koskela8, I. Koskinen9, V. Moilanen10, C. Nielsen11, U. Nieminen1, H. Nuutinen12, M. Heikkinen13, U.M. Suhonen14, J. Tillonen15, K. Utriainen16, I. Vihriälä17, C. Wennerström18,19, A. Borsi20, R. Nissinen21, M. Koivunen21, T. Sipponen1
1University of Helsinki and Helsinki University Hospital, Helsinki, Finland, 2Tampere University Hospital, Tampere, Finland, 3ESiOR Oy, Kuopio, Finland, 4Kanta-Häme Central Hospital, Hämeenlinna, Finland, 5North Karelia Central Hospital, Joensuu, Finland, 6South Karelia Central Hospital, Lappeenranta, Finland, 7Lapland Central Hospital, Rovaniemi, Finland, 8Oulu University Hospital, Oulu, Finland, 9Central Finland Central Hospital, Jyväskylä, Finland, 10Satakunta Central Hospital, Pori, Finland, 11Vaasa Central Hospital, Vaasa, Finland, 12Turku University Hospital, Turku, Finland, 13Kuopio University Hospital, Kuopio, Finland, 14Kainuu Central Hospital, Kajaani, Finland, 15Päijät-Häme Central Hospital, Lahti, Finland, 16Turku University Hospital Salo Hospital, Salo, Finland, 17Central Ostrobothnia Central Hospital, Kokkola, Finland, 18Janssen Cilag, Medical Affairs, Solna, Sweden, 19Department of Epidemiology Research, Statens Serum Institut, Copenhagen, Denmark, 20Janssen Cilag Limited, Emea Hemar, High Wycombe, UK, 21Medical Affairs, Janssen Cilag Oy, Espoo, Finland
Background
Real-life long-term evidence on ustekinumab treatment in patients with Crohn’s disease (CD) is limited. We performed a retrospective non-interventional nation-wide chart review study of dosing and long-term clinical outcomes in Finnish CD patients treated with ustekinumab (FINUSTE2, EUPAS30920).
Methods
FINUSTE2 was carried out in 17 Finnish centres. Eligible patients were adults with CD, receiving an intravenous (IV) first dose of ustekinumab during 2017 or 2018. Data on disease activity, dosage, and concomitant medications were collected at baseline, 16 weeks, and 1 year from treatment initiation. All measurements on ustekinumab trough concentrations (TC) were recorded.
Results
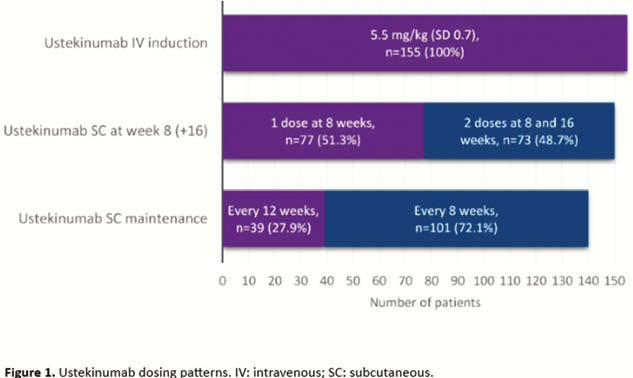
The study included 155 patients (48% female) with a mean age of 44 and disease duration of 14 years. The disease was stricturing or penetrating in 69% of patients, 59% had prior CD-related surgeries, and 96% had a treatment history of at least one biologic agent. After one IV dose and one to two subcutaneous (SC) doses at 8 to 16 weeks, 140 patients (93%) continued to maintenance treatment with SC ustekinumab, of which nearly three-quarters with a dosage interval of 8 weeks (Figure 1). Of 93 patients with a follow-up of at least 1 year, 77 were still on ustekinumab. During follow-up, 55 patients (39%) had their ustekinumab dose adjusted, mostly (
Conclusion
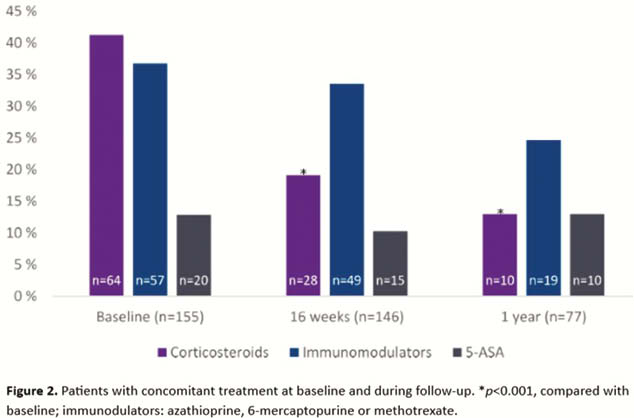
In this nationwide real-life study, treatment with ustekinumab in patients with longstanding and complicated CD was persistent and allowed for significant corticosteroid tapering. A vast majority started the maintenance treatment with an 8-week dosage interval and nearly one-third of all patients required a dose increase, suggesting a highly refractory disease phenotype. The lack of detected antidrug antibodies during follow-up indicates low immunogenicity for ustekinumab.


