DOP38 Upadacitinib Therapy Reduces Ulcerative Colitis Symptoms as Early as Day 1
Vermeire, S.(1);Colombel, J.F.(2);Takeuchi, K.(3);Gao, X.(4);Panaccione, R.(5);Danese, S.(6);Dubinsky, M.(7);Schreiber, S.(8);Ilo, D.(9);Finney-Hayward, T.(9);Zhou, W.(9);Phillips, C.(9);Yao, X.(9);Zhou, Q.(9);Loftus, E.(10);
(1)University Hospital Leuven, none, Leuven, Belgium;(2)Icahn School of Medicine at Mount Sinai, none, New York City, United States;(3)Tsujinaka Hospital Kashiwanoha- Kashiwa, Division of Gastroenterology and Hepatology, Chiba, Japan;(4)The Sixth Affiliated Hospital of Sun Yat-sen University, Department of Gastroenterology, Guangzhou, China;(5)University of Calgary, Division of Gastroenterology and Hepatology, Alberta, Canada;(6)IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Department of Gastroenterology and Endoscopy, Milan, Italy;(7)Icahn School of Medicine at Mt Sinai, Department of Pediatric Gastroenterology, New York, United States;(8)University Hospital Schleswig-Holstein, none, Kiel, Germany;(9)AbbVie- Inc, none, North Chicago, United States;(10)Mayo Clinic College of Medicine, Division of Gastroenterology and Hepatology, Rochester, United States;
Background
Upadacitinib (UPA), an oral, reversible JAK inhibitor engineered for increased selectivity for JAK1 over JAK2, JAK3, or tyrosine kinase 2 (TYK2), demonstrated significantly greater efficacy compared with placebo (PBO) for induction of remission in patients with moderately to severely active ulcerative colitis (UC) in two phase 3 trials, U-ACHIEVE Induction (NCT02819635) and U-ACCOMPLISH (NCT03653026). This analysis evaluated the efficacy of UPA on early symptomatic improvement for the first 14 days, using pooled data from U-ACHIEVE Induction and U-ACCOMPLISH.
Methods
U-ACHIEVE and U-ACCOMPLISH were multicentre, double-blind, PBO-controlled trials that enrolled patients who have had moderately to severely UC with an Adapted Mayo Score of 5 to 9 points and centrally reviewed endoscopy subscore of 2 to 3. A total of 998 patients were randomized to receive UPA 45mg once daily (QD) (n=658) or PBO (n=328) for 8 weeks (wks) in a 2:1 ratio. First dose of study drug was administered on Day 0. Improvement in symptoms including stool frequency subscore (SFS), rectal bleeding subscore (RBS), abdominal pain, and bowel urgency were analysed from daily symptom diary data. Multivariate regression analysis was used to determine if early changes in UC symptoms could be used to evaluate a potential correlation with patients’ likelihood of achieving clinical response or clinical remission per Adapted Mayo score at the end of induction.
Results
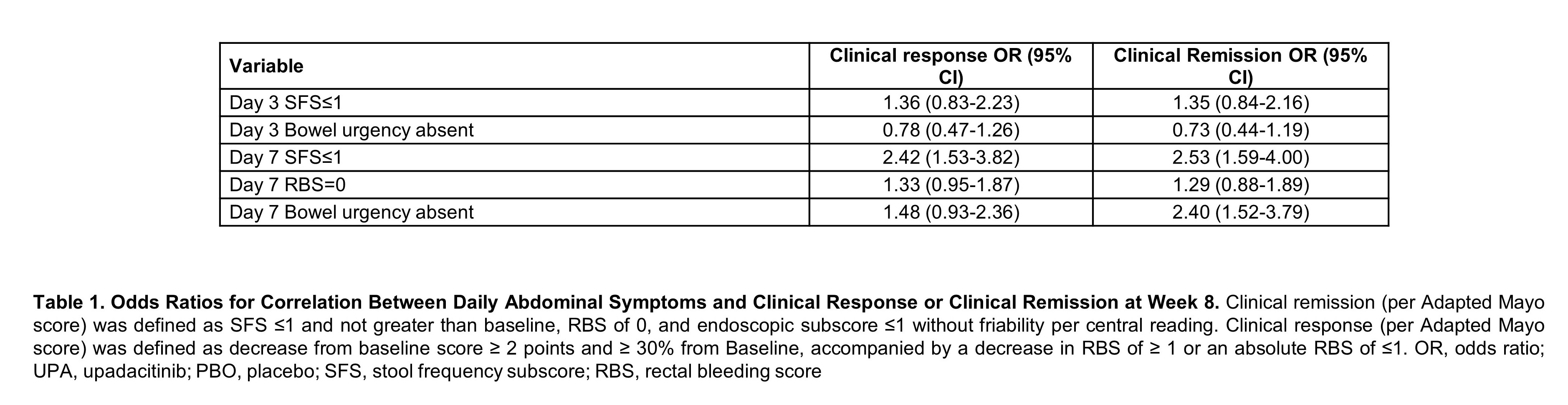
Baseline characteristics were similar between both treatment groups. Patients treated with UPA 45 mg QD experienced significant improvement in daily symptoms, with significantly more subjects achieving SFS≤1 (p<0.001), RBS of 0 (p<0.05), and SFS of 0 (p<0.05) as early as day 1 and maintained through day 14 (Figure 1). A significantly higher percentage of patients who received UPA 45 mg QD compared to PBO, achieved abdominal pain=0 and the absence of bowel urgency within 3 days of beginning treatment through day 14 (p<0.05). Multivariate analysis revealed that patients who achieved day 7 SFS≤1 (OR 2.42, 95% CI, 1.53-3.82) were more likely to attain clinical response (Table). Patients who attained day 7 SFS≤1 (OR 2.53, 95% CI 1.59-4.00) or day 7 bowel urgency absent (OR 2.40, 95% CI 1.52-3.79) were more likely to achieve clinical remission at week 8.
Conclusion
UPA 45 mg QD significantly improved UC symptoms as early as day 1, providing patients with rapid symptom relief. Patients who achieved early symptom improvement were more likely to attain clinical remission or clinical response at week 8. [Clinicaltrials.gov, U-ACHIEVE Induction (NCT02819635) and U-ACCOMPLISH (NCT03653026)]


