DOP39 The first prospective, multicentre, randomised controlled trial on discontinuation of infliximab in ulcerative colitis in remission; endoscopic normalisation does not guarantee successful withdrawal
T. Kobayashi1, S. Motoya2, S. Nakamura3, T. Yamamoto4, M. Nagahori5, S. Tanaka6, T. Hisamatsu7, F. Hirai8, H. Nakase9, K. Watanabe10, T. Matsumoto11, M. Tanaka12, T. Abe13, Y. Suzuki14, M. Watanabe5, T. Hibi1, HAYABUSA
1Center for Advanced IBD Research and Treatment, Kitasato Institute Hospital, Kitasato University, Tokyo, Japan, 2IBD Center, Sapporo Kosei General Hospital, Sapporo, Japan, 3Department of Inflammatory Bowel Disease, Hyogo College of Medicine, Nishinomiya, Japan, 4Yokkaichi Hazu Medical Centre, IBD Centre, Yokkaichi, Japan, 5Department of Gastroenterology and Hepatology, Tokyo Medical and Dental University, Tokyo, Japan, 6Department of Endoscopy and Medicine, Hiroshima University, Hiroshima, Japan, 7Department of Gastroenterology and Hepatology, School of Medicine, Kyorin University, Mitaka, Japan, 8Department of Gastroenterology, Faculty of Medicine, Fukuoka University, Fukuoka, Japan, 9Department of Gastroenterology and Hepatology, Sapporo Medical University School of Medicine, Sapporo, Japan, 10Department of Intestinal Inflammation Research, Hyogo College of Medicine, Nishinomiya, Japan, 11Division of Gastroenterology, Department of Internal Medicine, Iwate Medical University, Morioka, Japan, 12Department of Pathology, Hirosaki Municipal Hospital, Morioka, Japan, 13Department of Data Science, Yokohama City University, Yokohama, Japan, 14IBD Center, Toho University Sakura Medical Center, Sakura, Japan
Background
Anti-tumour necrosis factor (TNF)-α agents are the mainstay of the long-term treatments for refractory ulcerative colitis (UC). However, there is no prospective randomised controlled trial evaluating if anti-TNF-α agents can be discontinued in UC patients in remission.
Methods
Patients with UC maintained in clinical remission with infliximab (IFX) were prospectively enrolled from 23 specialist centres. Patients confirmed to be in (1) clinical remission for > 6 months, (2) steroid-free and (3) Mayo endoscopic subscore (MES) of 0 or 1 were randomised with stratified factors (MES and use of immunomodulators) into two groups (continue or discontinue IFX) in 1:1. The biopsy was taken from the rectum and Nancy histological Index (NI) was centrally scored at randomisation. The primary endpoint was remission rate at week 48 in full analysis set (FAS). Factors associated with remission at Week 48 were evaluated by logistic regression adjusted for the treatment group. Efficacy and safety of retreatment with IFX after relapse were also evaluated.
Results
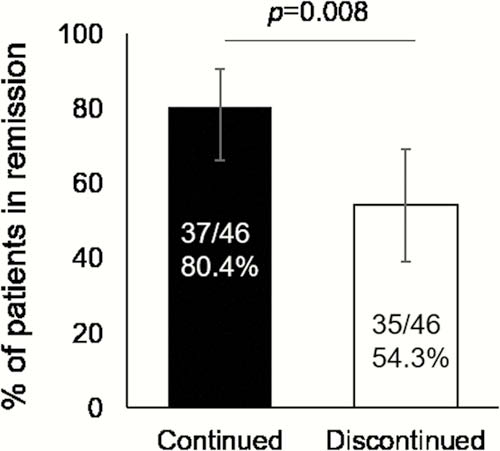
A total of 92 patients were included in the FAS and the remission rates at week 48 were 80.4% (95% CI: 66.1–90.6) and 54.3% (95% CI: 39.0–69.1) in IFX-continued and IFX-discontinued groups, respectively (
Conclusion
This first prospective multicentre randomised controlled trial confirmed that discontinuation of maintenance IFX resulted in the increased risk of relapse but retreatment was effective in UC. Endoscopic normalisation was not sufficient for the successful discontinuation of IFX.


