DOP42 Impact of discontinuing thiopurines at anti-TNF initiation in inflammatory bowel disease: A nationwide Danish cohort study
S. Bohn Thomsen1, R. Ungaro2, K. Allin3, G. Poulsen1, A. Mikael1, J.F. Colombel2, T. Jess1
1Department of Epidemiology Research, Statens Serum Institut, Copenhagen, Denmark, 2Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, USA, 3Center for Clinical Research and Prevention, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark
Background
The impact of discontinuing vs. continuing thiopurines at anti-TNF initiation in thiopurine experienced patients with inflammatory bowel disease (IBD) is unclear.
Methods
We used the nationwide Danish registers to establish a national cohort of patients with IBD who received thiopurines prior to initiating anti-TNF during 2003–2014. We compared patients who discontinued vs. continued thiopurine within 90 days of anti-TNF initiation. Our primary outcome was a composite of any clinical event: corticosteroids, hospitalisation, surgery, or death. We used Cox regression models to calculate adjusted hazard ratios (aHR) with 95% confidence intervals (CI). Analyses were adjusted for sex, diagnosis-age, IBD-subtype, disease duration, calendar year, pre-anti-TNF thiopurine duration, and past disease severity including hospitalisations the past year, surgery past 5 years, and corticosteroid use the past year.
Results
Of 6998 anti-TNF exposed, 1602 patients (Crohn’s disease,
IR; incidence rate, HR; hazard ratio, CI; confidence interval, IBD; inflammatory bowel disease.
Figure. Cumulative incidence of an outcome in patients who discontinued vs. continued thiopurines after anti-TNF initiation. (A) Cumulative incidence of the composite outcome (corticosteroids, major surgery, hospitalisation, or death). (B) Cumulative incidence of major surgery. (C) Cumulative incidence of hospitalisation. (D) Cumulative incidence of corticosteroids.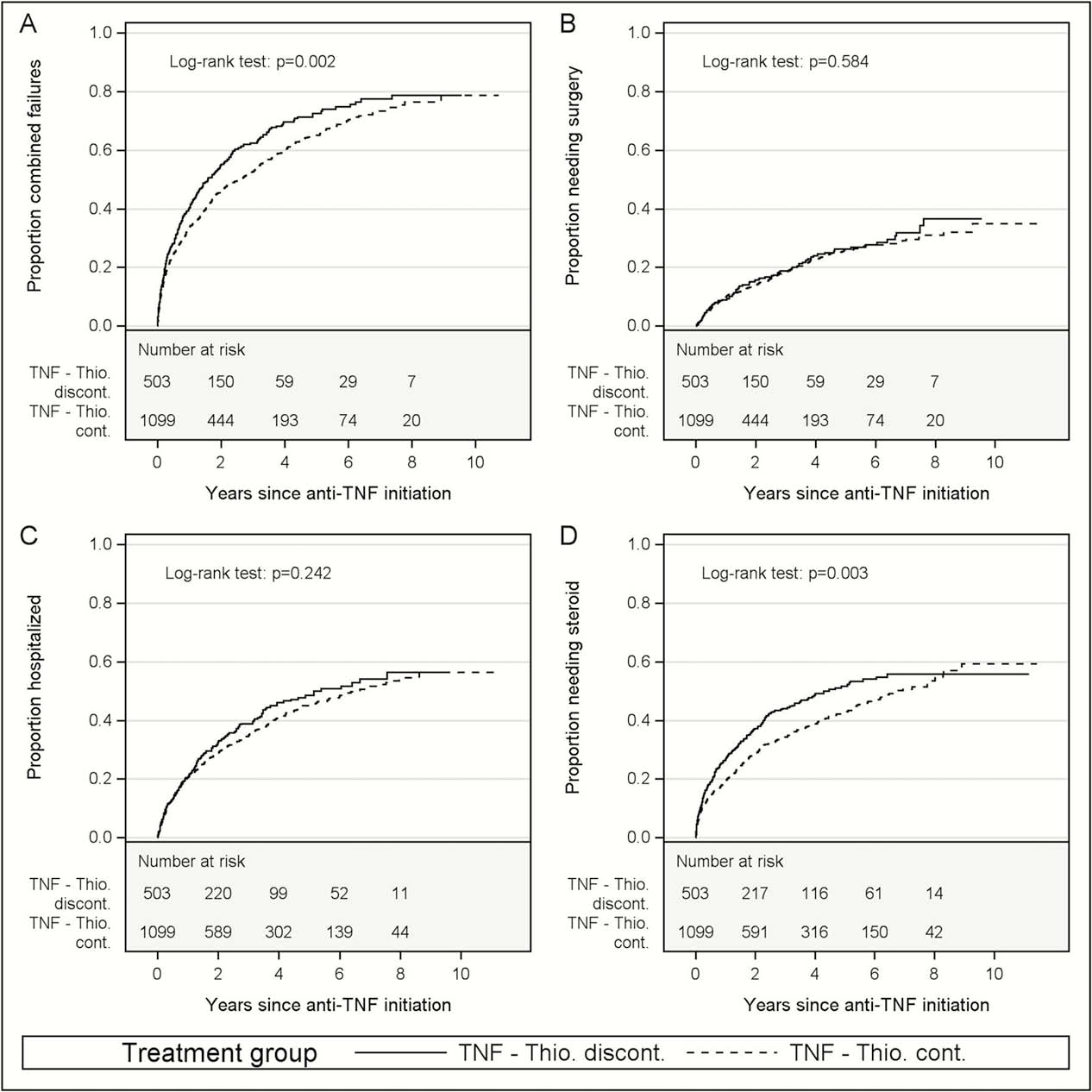
Conclusion
In our nationwide cohort study of patients with IBD, we found that continuing thiopurines after anti-TNF initiation impacted the outcome favourably, especially regarding corticosteroid use. Further studies are warranted to investigate this central clinical question.


