DOP52 The phenotype of NOD-scid IL-2Rγnull mice reconstituted with peripheral blood mononuclear cells from patients with Crohn’s disease or ulcerative colitis reflects the respective disease
A. Unterweger1, J. Caesar1, P. Winkelmann1, A. Rüscher1, M. Seuß1, S. Breiteneicher5, J. Stallhofer5, F. Beigel5, M. Siebeck1, R. Gropp1
1Hospital of the Ludwig Maximilian Universität, General Visceral und Transplantation Surgery, München, Germany, 5Hospital of the Ludwig Maximilian Universität, Department of Medicine II, München, Germany
Background
Recently, we have developed a mouse model that relies on NOD-scid IL-2Rγnull (NSG) mice reconstituted with peripheral blood mononuclear cells (PBMC) derived from patients with ulcerative colitis (UC, NSG-UC). In this model, symptoms of UC are induced by rectal challenge with ethanol. The objective of the study was to adapt this model to Crohn’s disease and to compare the phenotypes of the NSG-UC and NSG-CD mouse models.
Methods
NSG mice were reconstituted with PBMC from UC (
Results
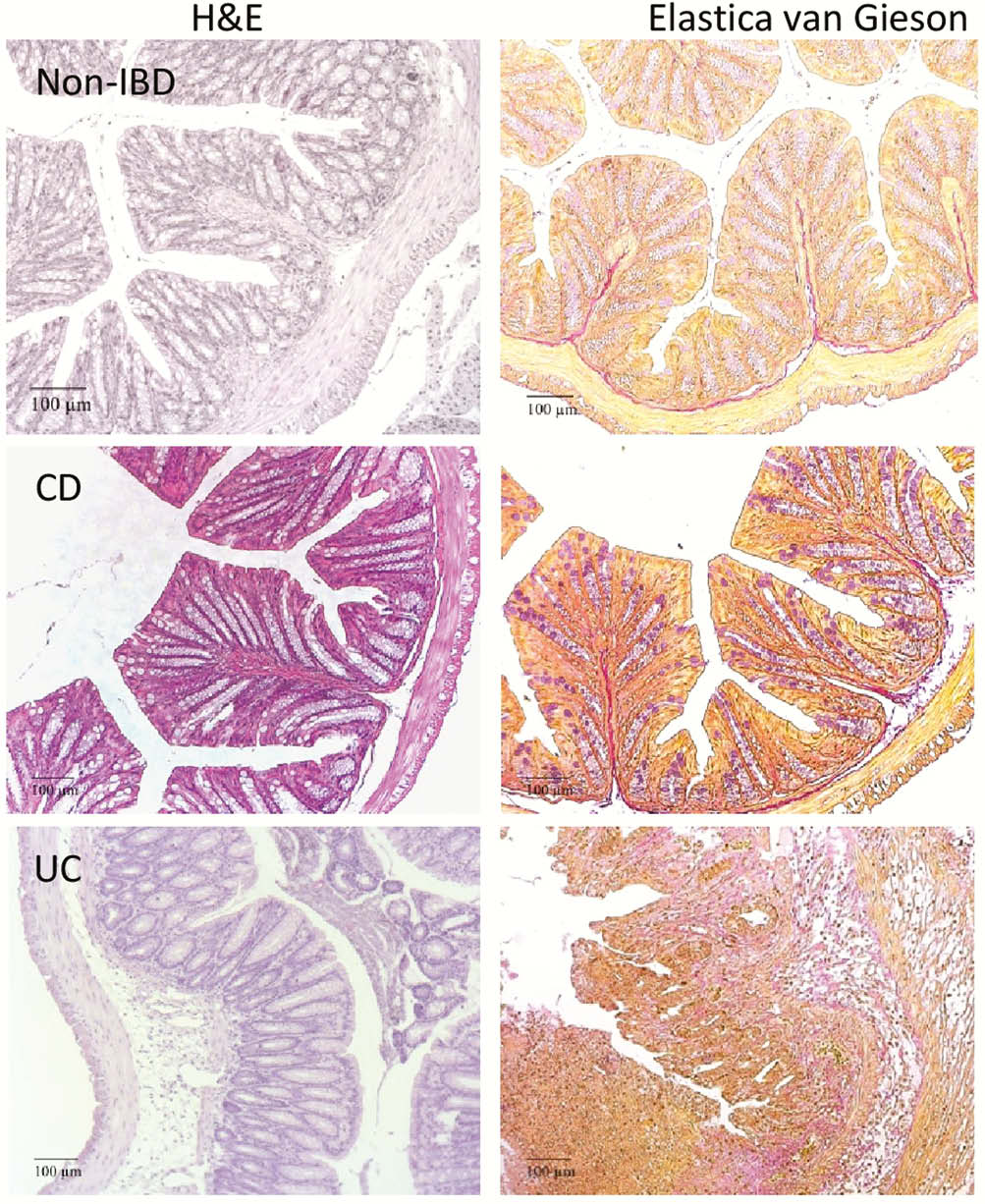
The pathological phenotype was markedly different in NSG-UC mice as compared with NSG-CD mice. Firstly, histological analysis revealed that NSG-UC mice exhibited more of a pro-inflammatory phenotype as indicated by a severe influx of inflammatory cells, oedema, crypt loss, crypt abscesses and epithelial hyperplasia. In contrast, NSG-CD mice displayed crypt loss and goblet cell atrophy and pronounced fibrosis indicating ongoing wound healing processes.
These observations were corroborated by frequencies of splenic and colonic leucocytes. Antigen experienced CD4+ T (CD45RO+) cells and switched B cells (CD19+ CD27+ IgD-) were significantly increased in NSG-UC mice, whereas significantly higher levels of experienced CD8+ T cells and M1 (CD64+), M2 (CD163+) CD14+ monocytes and unswitched B cells (CD19+ CD27+ IgD+) indicated a monocyte driven inflammation in NSG-CD mice.
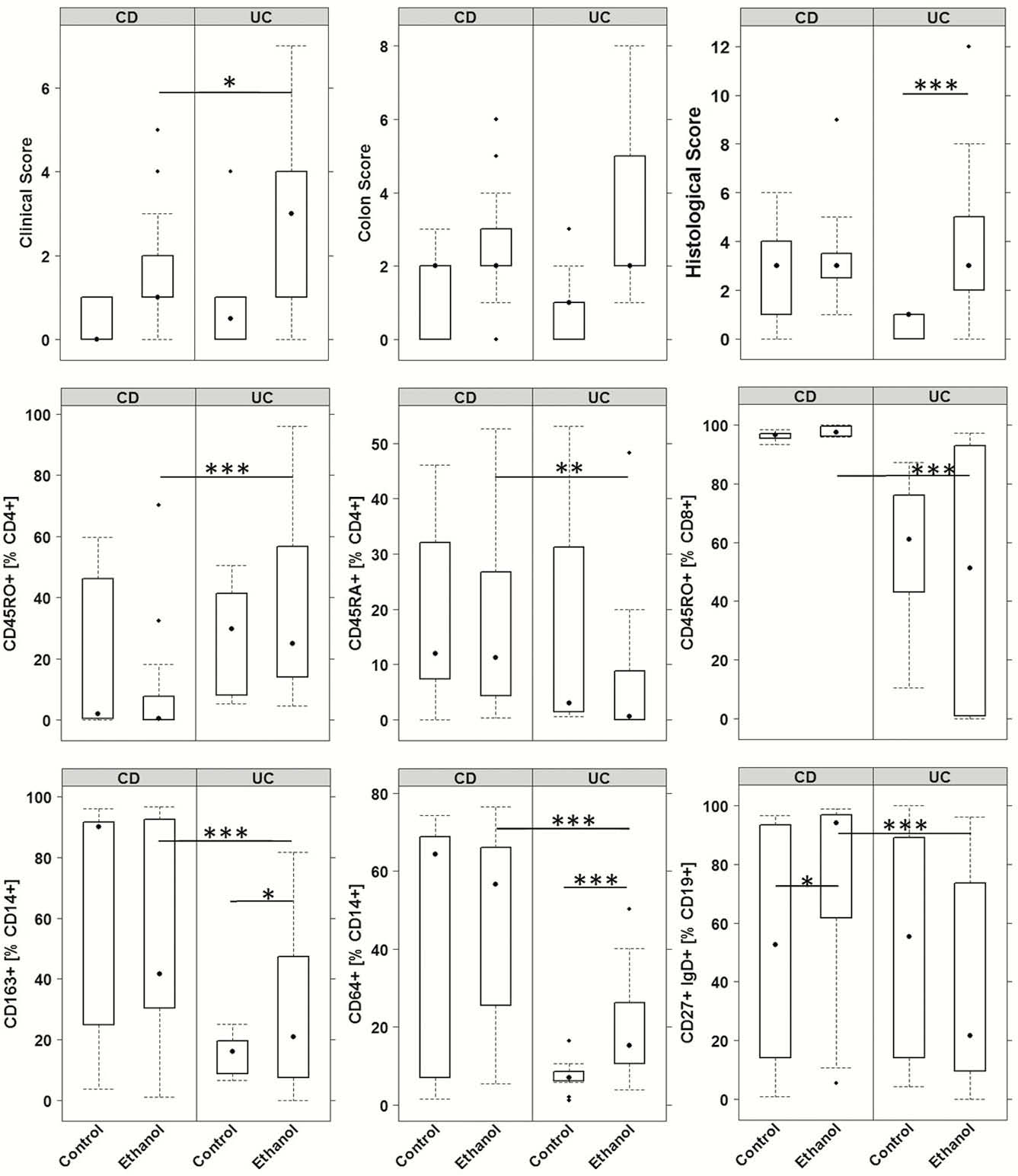
This observation was also reflected in the colon of mice. Inflammation in UC was characterised by increased frequencies of neutrophils, activated CD4+ T cells (CD69+) and increased levels of CRP and MCP-3, whereas NSG-CD mice were signified by increased frequencies of M2 and M1 monocytes and of TGFß levels. In contrast to NSG-CD mice, NSG-UC mice also displayed higher serum levels of IL-6. Secondly, the impact of ethanol was more pronounced in the NSG-UC mice. In NSG-CD mice, the challenge did not evoke significant differences as compared with unchallenged control mice.
Conclusion
The comparison of pathological phenotypes of the NSG-UC and NSG-CD mouse models revealed differences, some of which reflect the respective human disease. The NSG-UC and NSG-CD mouse models may constitute powerful tools to get a better understanding of the different inflammatory processes in UC and CD.


