DOP56 Ustekinumab maintained clinically meaningful improvement in health-related quality of life in patients with moderate to severe ulcerative colitis: Results from the UNIFI long-term extension
W.J. Sandborn1, D.S. Rowbotham2, R.W.L. Leong3, C. Han4, Y. Zhou5, H. Zhang5, J. Johanns5, C. Marano6, S. Danese7, UNIFI Investigators
1Department of Gastroenterology, University of California San Diego, La Jolla, USA, 2Department of Gastroenterology and Hepatology, Auckland City Hospital, Auckland, New Zealand, 3Department of Gastroenterology, Concord and Macquarie University Hospitals, Sydney, Australia, 4Strategic Market Access, Janssen Global Services- LLC, Malvern, USA, 5Department of Clinical Biostats, Janssen Research and Development- LLC, Spring House, USA, 6Department of Immunology, Janssen Research and Development- LLC, Spring House, USA, 7Department of Gastroenterology, Humanitas Research Hospital, Milan, Italy
Background
The UNIFI maintenance study evaluated the safety and efficacy of subcutaneous (SC) ustekinumab (UST) in patients with moderately–severely active UC who had responded to IV UST induction. Previously, we reported that health-related quality of life (HRQoL) improvements achieved after UST induction were maintained through Week 44 with UST maintenance. Here, we evaluated HRQoL through Week 92 in patients who continued UST maintenance in the long-term extension (LTE).
Methods
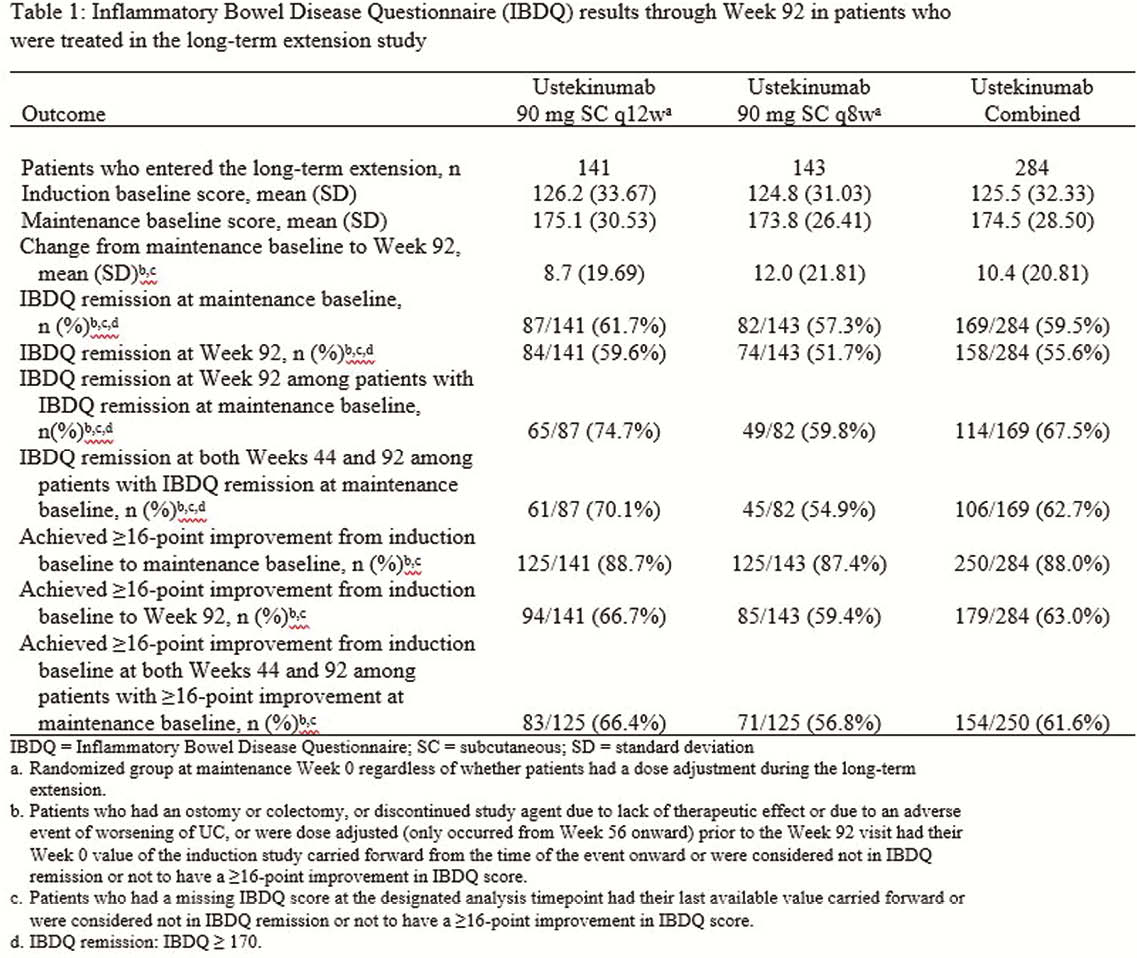
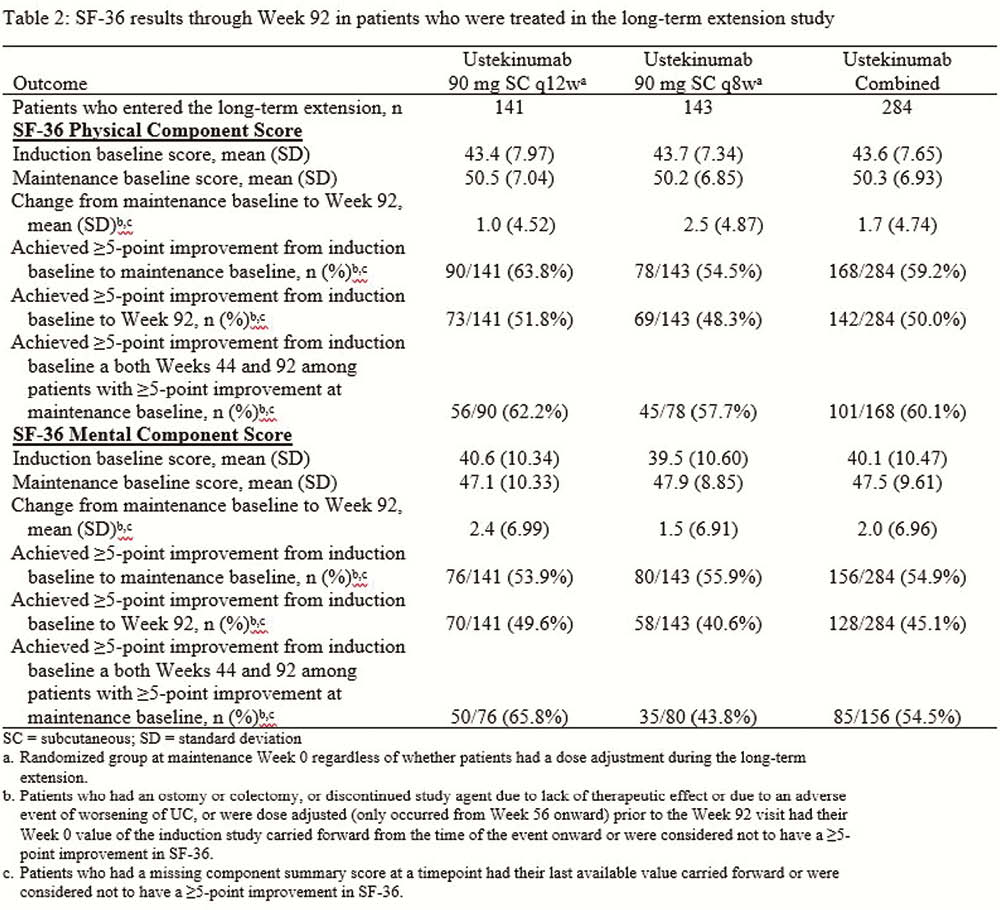
Patients who completed the maintenance study were eligible to continue their maintenance treatment regimen (placebo [PBO], UST90mg q12w, or UST90mg q8w) in the LTE if the investigator thought they could benefit from continued treatment. PBO patients discontinued from the LTE after the maintenance study was unblinded. Per investigator discretion, patients could receive a single dose adjustment (UST q12w to q8w or UST q8w to q8w [sham dose adjustment]) starting at Week 56. The Inflammatory Bowel Disease Questionnaire (IBDQ) is a 32-item questionnaire with a total score ranging from 32 to 224. Higher scores indicate better HRQoL, a score ≥170 indicates remission, and a change ≥16 was defined as clinically meaningful. General health was assessed using SF-36. A change ≥5 points in physical and mental component scores was defined as clinically meaningful. In this analysis, patients who dose adjusted were considered treatment failures.
Results
Most patients who received UST in the LTE maintained the improvements in IBDQ and SF-36 that were achieved after induction (Tables 1 and 2) through Week 92. Overall, 158 of 284 patients (55.6%) who received UST were in IBDQ remission at Week 92, and 114 of 169 patients (67.5%) who were in IBDQ remission at maintenance baseline were in IBDQ remission at Week 92. Of the 284 patients, 179 (63.0%) achieved a ≥16-point improvement in IBDQ score from induction baseline to Week 92. Of 250 patients who achieved a ≥16-point improvement from induction baseline to maintenance baseline, 154(61.6%) maintained a ≥16-point improvement at both Weeks 44 and 92. For SF-36, 142 of 284 patients (50.0%) had a ≥5-point improvement from induction baseline to Week 92 in the physical component score, while 128 of 284 (45.1%) had a ≥5-point improvement in the mental component score.
Conclusion
The majority of patients who were treated with UST in the LTE generally maintained improvements in IBDQ and SF-36 scores that were achieved after IV induction.


