DOP58 Tofacitinib for ulcerative colitis: Results of the ICC Registry, a nationwide prospective observational cohort study
V.B. Biemans1, J.A.M. Sleutjes2, A.C. de Vries2, A.G. Bodelier3, G. Dijkstra4, B. Oldenburg5, M. Löwenberg6, A.A. van Bodegraven7, A.E. van der Meulen-de Jong8, N.K. de Boer9, N. Srivastava10, R.L. West11, T. Römkens12, C.S. Horjus Talabur Horje13, J.M. Jansen14, J. Hoekstra3, R.K. Weersma4, F.D. van Schaik5, F. Hoentjen1, M.J. Pierik15, Dutch Initiative on Crohn and Colitis (ICC)
1Department of Gastroenterology and Hepatology, Radboudumc, Nijmegen, The Netherlands, 2Department of Gastroenterology and Hepatology, Erasmus Medical Center, Rotterdam, The Netherlands, 3Department of Gastroenterology and Hepatology, Amphia Hospital, Breda, The Netherlands, 4Department of Gastroenterology and Hepatology, University Medical Center Groningen, Groningen, The Netherlands, 5Department of Gastroenterology and Hepatology, University Medical Center Utrecht, Utrecht, The Netherlands, 6Department of Gastroenterology and Hepatology, Amsterdam University Medical Center- AMC, Amsterdam, The Netherlands, 7Department of Gastroenterology and Hepatology, Zuyderland Medical Center, Sittard, The Netherlands, 8Department of Gastroenterology and Hepatology, Leiden University Medical Center, Leiden, The Netherlands, 9Department of Gastroenterology and Hepatology, Amsterdam University Medical Center, Vrije Universiteit, Amsterdam, The Netherlands, 10Department of Gastroenterology and Hepatology, Haaglanden Medical Center, Den Haag, The Netherlands, 11Department of Gastroenterology and Hepatology, Sint Franciscus Hospital, Rotterdam, The Netherlands, 12Department of Gastroenterology and Hepatology, Jeroen Bosch Hospital, ‘s-Hertogenbosch, The Netherlands, 13Department of Gastroenterology and Hepatology, Rijnstate Hospital, Arnhem, The Netherlands, 14Department of Gastroenterology and Hepatology, OLVG Hospital, Amsterdam, The Netherlands, 15Department of Gastroenterology and Hepatology, Maastricht University Medical Center, Maastricht, The Netherlands
Background
Tofacitinib is a janus kinase 1 and 3 inhibitor approved for the treatment of ulcerative colitis (UC). Real-world evidence is needed to evaluate the effectiveness, usage and safety in daily practice.
Methods
UC patients in whom tofacitinib was started in 15 hospitals (8 academic, 7 non-academic) participated in the
Results
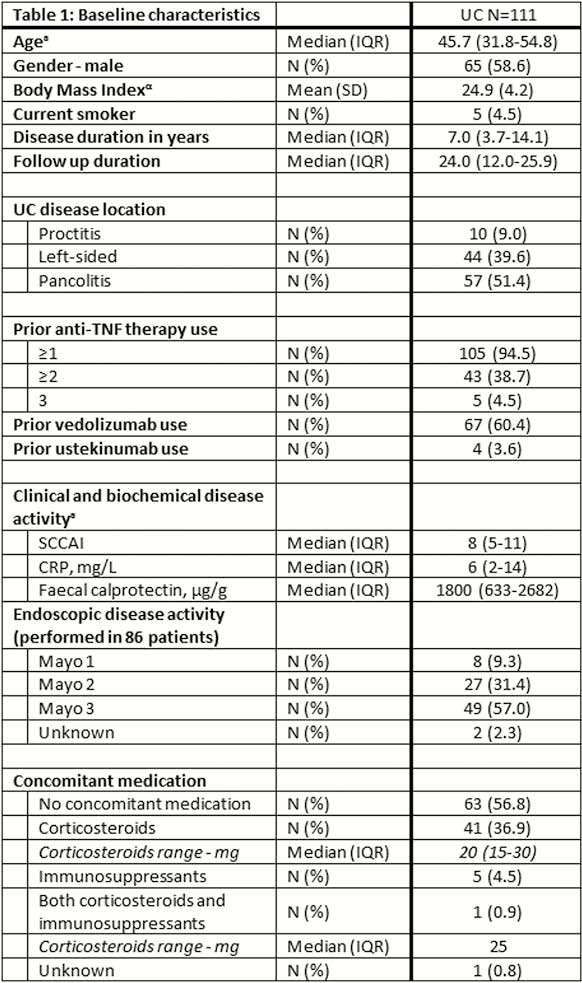
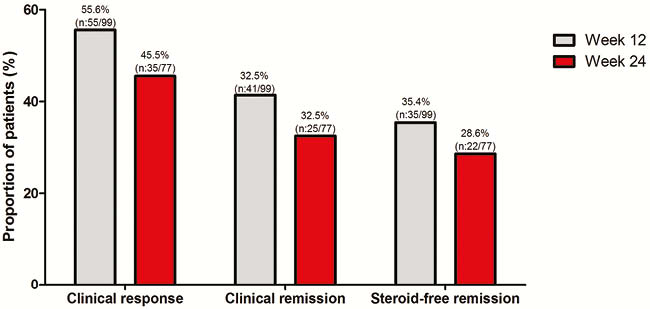
In total, 111 UC patients (95% anti-TNF, 60% vedolizumab, 4% ustekinumab exposed) were followed for a median of 24 weeks (IQR 12–26). All patients had both active clinical and objective disease (SCCAI 8 (IQR 5–11), FCP 1800µg/g (IQR 633–2682)). Corticosteroid-free clinical, biochemical, and combined corticosteroid-free clinical and biochemical remission rate at week 24 was 29%, 25%, and 19%, respectively. Endoscopic remission was achieved in 21% of patients (
Conclusion
Tofacitinib is an effective treatment for UC after anti-TNF and/or vedolizumab failure. We did observe a relatively high rate of adverse events in this refractory UC cohort.


