DOP63 Real World Effectiveness, Safety and Pharmacokinetics of Switching Intravenous Vedolizumab Maintenance treatment to Subcutaneous Vedolizumab Therapy for Inflammatory Bowel Disease
Volkers, A.(1);Straatmijer, T.(1);Duijvestein, M.(2);Sales, A.(1);Levran, A.(1);van Schaik, F.(3);Jeroen, M.(4);Gecse, K.(1);Ponsioen, C.(1);Grootjans, J.(1);Hanzel, J.(1);Tack, G.(5);Jansen, J.(6);Hoentjen, F.(7);de Boer, N.(1);van der Marel, S.(8);Dijkstra, G.(9);Oldenburg, B.(3);Löwenberg, M.(1);van der meulen, A.(4);D'Haens, G.(1);
(1)Amsterdam UMC- location AMC, Department of Gastroenterology and Hepatology, Amsterdam, The Netherlands;(2)Radboud University Medical Center, Department of Gastroenterology and Hepatology, Nijmegen, The Netherlands;(3)University Medical Center Utrecht, Department of Gastroenterology and Hepatology, Utrecht, The Netherlands;(4)Leiden University Medical Center, Department of Gastroenterology and Hepatology, Leiden, The Netherlands;(5)Medical Center Leeuwarden, Department of Gastroenterology and Hepatology, Leeuwarden, The Netherlands;(6)Onze Lieve Vrouwe Gasthuis, Department of Gastroenterology and Hepatology, Amsterdam, The Netherlands;(7)University of Alberta, Division of Gastroenterology- Department of Medicine-, Edmonton, Canada;(8)Haaglanden Medical Center, Department of Gastroenterology and Hepatology, Amsterdam, The Netherlands;(9)University Medical Center Groningen, Department of Gastroenterology and Hepatology, Groningen, The Netherlands; on behalf of the IBD center of Amsterdam and the Dutch Initiative on Crohn and Colitis (ICC)
Background
Subcutaneous (SC) formulation of vedolizumab (VDZ) is available for Crohn’s disease (CD) and ulcerative colitis (UC). We assessed the efficacy, safety, and pharmacokinetic (PK) profiles of patients with inflammatory bowel diseases (IBD) who switched from intravenous (IV) to SC VDZ treatment in two prospective, real world cohorts.
Methods
The primary cohort is an ongoing open-label, real life, prospective single centre cohort study. As a validation cohort, we used the Initiative on Crohn and Colitis (ICC) registry, a prospective, observational, nationwide registry including patients switching from IV to SC VDZ. In both cohorts, patients receiving IV VDZ maintenance for >4 months were offered to switch treatment to SC VDZ, 108 mg every 2 weeks. In the primary cohort, assessment of clinical, biochemical and PK parameters took place at baseline, at approximately 10 weeks following the switch and at the physician’s discretion thereafter. In the ICC cohort, follow up visits were at week 12 and 24. The primary endpoint was the proportion of patients discontinuing SC VDZ at week 24.
Results
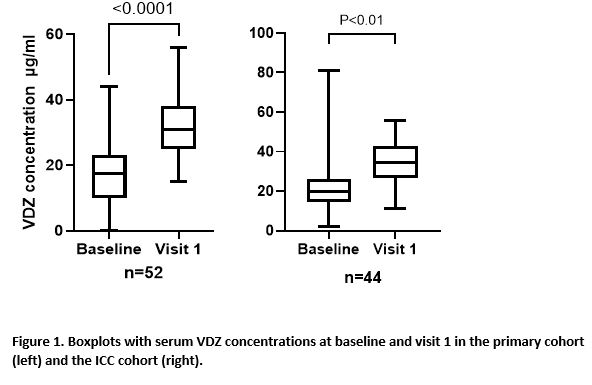
In total, 78 (50 CD (64%) and 28 UC (36%)) and 54 patients (29 CD (54%) and 25 UC (46%)) were included in the primary and ICC cohort respectively (table 1). During follow up, 8 (10.3%) of the primary cohort and 6 (11.1%) patients of the ICC cohort stopped VDZ SC during follow-up time till week 24, after a median treatment duration of 18 (IQR=5-19) and 10 (IQR=7-15) weeks, respectively. Treatment withdrawal was most often caused by adverse events (AE), in total for 8 out of 132 patients (6%) (table 2). Four patients had loss of response to SC VDZ. Three of these patients had biochemical disease activity at initiation of SC therapy. Reported AEs included headache and injection related reactions. The median VDZ concentration increased from 11 ug/mL (IQR=9.4-20) to 28 ug/mL (IQR=24.3-31.2, p<0.0001) and from 20 ug/mL (14.3-26.3) to 34.6 ug/mL (26.8-42.9) (p<0.01), between baseline and visit 1 in the primary and validation cohort, respectively (figure 1).


Conclusion
The present abstract reports real world experience of switching IV to SC VDZ maintenance treatment in IBD patients in two observational Dutch cohorts. VDZ concentrations were significantly higher after the switch to SC VDZ. A switch from IV to SC VDZ appears to be effective and safe. However, a proportion of patients switched back to IV VDZ due to injection related AEs.


