DOP69 Tofacitinib in ulcerative colitis: Early ‘real-world’ experience from four UK tertiary centres
D. Chee1, S. Honap2, T.P. Chapman3, A.J. Kent4, M. Patel4, B. Hayee4, P. Dubois4, L. Medcalf4, E. Sharma5, Y. Begum5, S. Ray2, J. Kennedy3, S. Cripps6, C. Elworthy7, A. Walsh3, J.R. Goodhand1, T. Ahmad1, J. Satsangi3, N.A. Kennedy1, P. Irving2, LEO (London, Exeter, Oxford) Consortium - Travis S, Brain O, Palmer R
1Department of Gastroenterology, Royal Devon and Exeter NHS Foundation Trust, Exeter, UK, 2Department of Gastroenterology, Guy’s and St Thomas’ NHS Foundation Trust, London, UK, 3Translational Gastroenterology Unit, John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust, Oxford, UK, 4Department of Gastroenterology, King’s College Hospital NHS Foundation Trust, London, UK, 5Department of Pharmacy, Guy’s and St Thomas’ NHS Foundation Trust, London, UK, 6Pharmacy Department, John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust, Oxford, UK, 7Department of Pharmacy, Royal Devon and Exeter NHS Foundation Trust, Exeter, UK
Background
Tofacitinib is a partially selective Janus kinase inhibitor that was approved for the treatment of refractory moderate to severe ulcerative colitis (UC) in 2018. We report the real-world clinical effectiveness and adverse effects of tofacitinib in UC.
Methods
We conducted a retrospective observational cohort study of tofacitinib-treated patients with UC between October 2018 to October 2019 from 4 UK centres. Disease activity was assessed using the Simple Clinical Colitis Activity Index (SCCAI) or Partial Mayo Score (PMS) depending on the study site. Response and remission were defined at week 8 and 26 as a reduction in SCCAI or PMS of ≥3, and SCCAI <3 or PMS <2, respectively. Corticosteroid-free remission was defined as remission with no corticosteroid use at the time of assessment irrespective of baseline corticosteroid status.
Results
We included 140 patients (65% M; median age 37y [range 16–81]) with a median disease duration of 5.5y (IQR 2.2–11.8). Forty-six per cent (65/140) were receiving corticosteroids at baseline and 83% (116/140) had previously received at least one biologic (62 anti-TNF, 4 vedolizumab, 50 both). Median (IQR) serum CRP and faecal calprotectin levels at baseline were 4 mg/l (1.6–15) and 540 µg/g (316–1175).
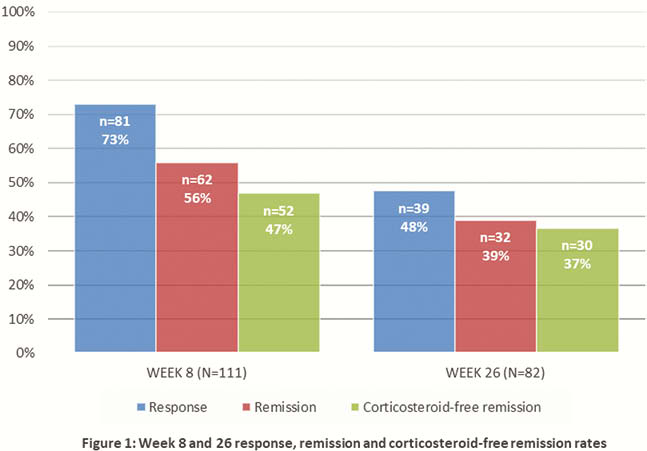
Response and remission rates were 73% (81/111) and 56% (62/111) respectively at week 8 (median ΔSCCAI of −3 [IQR -6 to -1], median ΔPMS −4 [−6 to −1]) and 48% (39/82) and 39% (32/82) respectively at week 26 (median ΔSCCAI −3 [IQR −7 to –1], median ΔPMS −4 [−6 to –1]). Steroid-free remission was seen in 47% (52/111) and 37% (30/82) patients at week 8 and 26. Patients with response or remission had a significantly lower CRP (
Conclusion
Clinical effectiveness and side-effect profile of tofacitinib in UC in this multi-centre real-world cohort were similar to that reported in the pivotal OCTAVE clinical trials.


