DOP73 Efficacy and safety of escalation to tofacitinib 10 mg BID for patients with UC following loss of response on 5 mg BID maintenance therapy: Results from OCTAVE open-label, long-term extension study
B.E. Sands1, A.C. Moss2, A. Armuzzi3, J.K. Marshall4, J.O. Lindsay5, W.J. Sandborn6, S. Danese7, M. Zeroncio8, I. Modesto9, L. Salese10, N. Lawendy10, H. Zhang10, C. Su10
1Icahn School of Medicine at Mount Sinai, Dr. Henry D. Janowitz Division of Gastroenterology, New York, NY, USA, 2Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA, USA, 3IBD Unit, Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy, 4Division of Gastroenterology, Department of Medicine, Farncombe Family Digestive Health Research Institute, McMaster University, Hamilton, Ontario, Canada, 5Department of Gastroenterology, The Royal London Hospital, Barts Health NHS Trust, London, UK, 6Division of Gastroenterology, University of California - San Diego, La Jolla, CA, USA, 7IBD Center, Department of Gastroenterology, Humanitas Research Hospital, Rozzano, Milan, Italy, 8Brazilian IBD Study Group, Avenida Brigadeiro Faria Lima, São Paulo, Brazil, 9Pfizer Inc., New York, NY, USA, 10Pfizer Inc., Collegeville, PA, USA
Background
Tofacitinib is an oral, small-molecule JAK inhibitor for the treatment of ulcerative colitis (UC). The efficacy and safety of tofacitinib have been demonstrated in patients with moderate to severe UC in three Phase 3 studies (OCTAVE Induction 1 and 2 [NCT01465763; NCT01458951]; OCTAVE Sustain [NCT01458574]) [1]. Here, we present updated efficacy and safety data of tofacitinib dose escalation in patients with UC participating in an ongoing, open-label, long-term extension (OLE) study (OCTAVE Open, NCT01470612) [2].
Methods
We present updated data from the dose-escalation subpopulation of the OLE study (as of May 2019; database not locked) comprising patients who achieved clinical response (CR) following 8 weeks of tofacitinib 10 mg twice daily (BID) induction therapy, entered OCTAVE Sustain receiving tofacitinib 5 mg BID, experienced treatment failure between Week 8 and Week 52, and subsequently entered OCTAVE Open with escalation to tofacitinib 10 mg BID. Treatment failure was defined as an increase of ≥3 points from maintenance study baseline total Mayo score, plus an increase of ≥1 point in both rectal bleeding subscore and centrally read endoscopic subscore (ES), and an absolute ES of ≥2 after ≥8 weeks of maintenance therapy. CR, mucosal healing (MH) and remission (R) were evaluated at Months 2, 12, 24 and 36 of OCTAVE Open (non-responder imputation and last observation carried forward [NRI-LOCF] and observed data). Safety was evaluated throughout the study.
Results
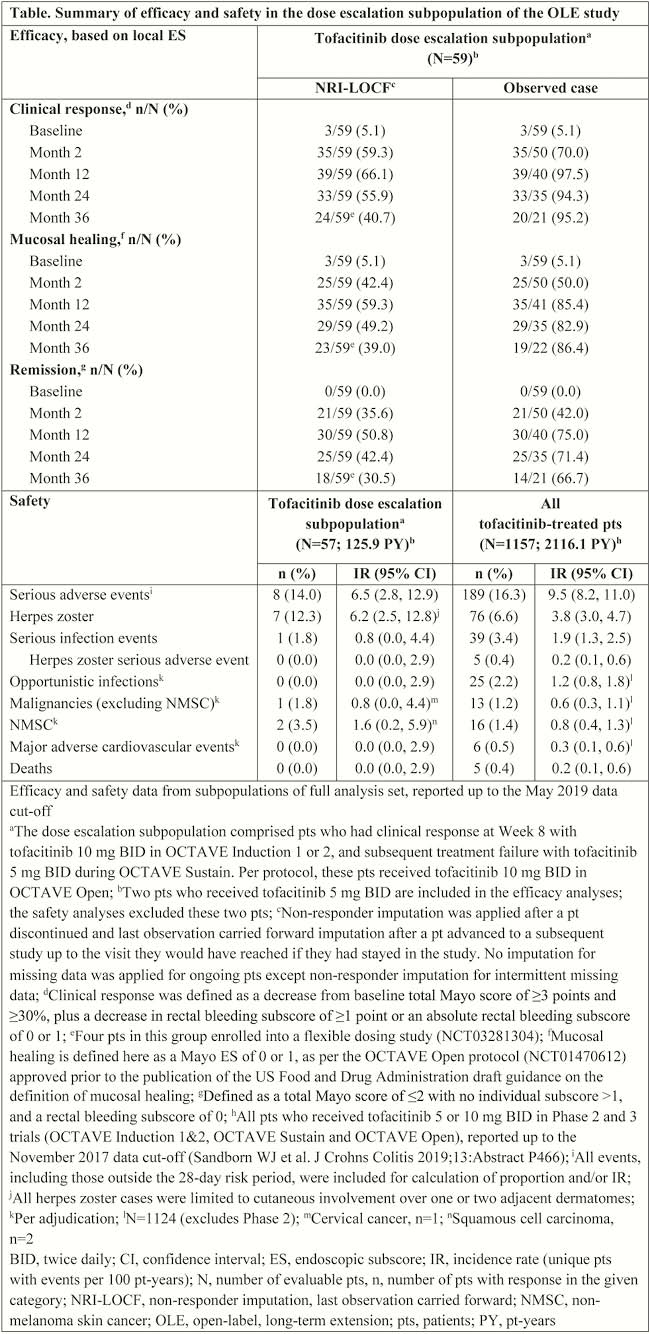
Of 944 patients enrolled in the OLE study, the dose escalation subpopulation comprised 59 patients. In these patients, CR, MH and R rates 36 months after dose escalation were, respectively, 40.7%, 39.0% and 30.5% for NRI-LOCF and 95.2%, 86.4% and 66.7% for observed data (Table). Of these 59 patients, 29 had prior tumour necrosis factor inhibitor (TNFi) failure; in these patients, CR, MH and R rates at Month 36 were, respectively, 51.7%, 51.7% and 41.4% for NRI-LOCF, and 100.0%, 92,3% and 75.0% for observed data. Incidence rates for safety events and pt-years’ exposure are reported in the table.
Conclusion
For most patients who lost initial CR to tofacitinib 10 mg BID induction therapy while on tofacitinib 5 mg BID maintenance therapy, including those with prior TNFi failure, dose-escalation back to 10 mg BID recaptured CR by Month 2 and was generally maintained over 3 years. The safety profile with tofacitinib 10 mg BID in the dose-escalation subpopulation was generally consistent with that in the overall study population, although there was a numerically higher rate of herpes zoster. These analyses are limited by low pt numbers and the absence of a comparator arm.
Sandborn WJ
Lichtenstein GR


