DOP76 Corticosteroid sparing effects of ustekinumab therapy for ulcerative colitis through 2 years: UNIFI long-term extension
L. Peyrin-Biroulet1, W. Sandborn2, B. Sands3, E. Scherl4, C. Marano5, C. O’Brien5, H. Zhang6, J. Johanns6, Y. Zhou6, M. Abreu7, R.P. Arasaradnam8, D.S. Rowbotham9, R.W.L. Leong10, S. Danese11, UNIFI Investigators
1Department of Gastroenterology, Nancy University Hospital, Nancy, France, 2Department of Gastroenterology, University of California - San Diego, La Jolla, USA, 3Department of Gastroenterology, Icahn School of Medicine at Mount Sinai, La Jolla, USA, 4Weill Cornell Medical Center, Jill Roberts Center for Inflammatory Bowel Disease, New York, USA, 5Department of Immunology, Janssen Research and Development- LLC, Spring House, USA, 6Department of Clinical Biostats, Janssen Research and Development- LLC, Spring House, USA, 7Department of Gastroenterology, University of Miami Miller School of Medicine, Miami, USA, 8Warwick Medical School, Department of Gastroenterology, University Hospital Coventry, Coventry, UK, 9Department of Gastroenterology and Hepatology, Auckland City Hospital, Auckland, New Zealand, 10Department of Gastroenterology, The University of New South Wales and Macquarie University, Sydney, Australia, 11Department of Gastroenterology, Humanitas Research Hospital, Milan, Italy
Background
Ustekinumab (UST) is an IL12/23 blocker approved for Crohn’s disease and ulcerative colitis (UC). In the UNIFI maintenance study of patients with moderate–severe UC, >90% of the patients who achieved clinical response or remission at week 44 were able to eliminate corticosteroids, an important goal of therapy. In this analysis, we describe the corticosteroid (CS) sparing effects of UST treatment through week 92 among patients who were treated in the UNIFI long-term extension (LTE).
Methods
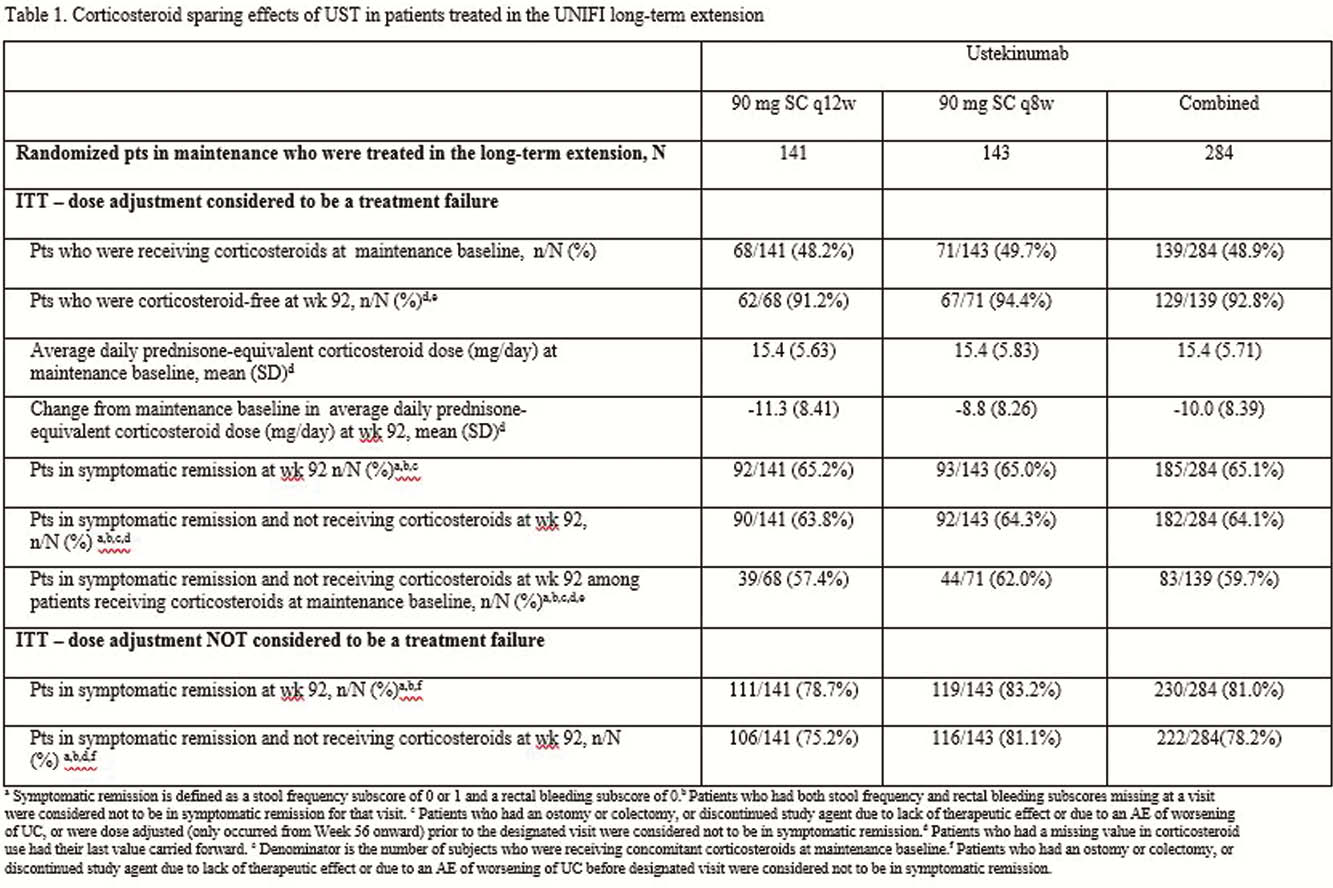
Responders to UST 8 weeks after IV induction entered the maintenance study and were randomised to UST 90mg SC (q12wks or q8wks) or PBO. Patients who did not respond to UST IV induction received a dose of UST 90mg SC at week 8 and were evaluated at week 16. Those in clinical response at week 16 (delayed responders), were not randomised on entry into the maintenance study and received UST 90mg q8wk. All patients who completed week 44 of the maintenance study were eligible to enter the LTE and continue to receive the same dose. Patients could receive UST dose adjustment during the LTE (q12wk to q8wk or q8wk to q8wk [sham dose adjustment]) from week 56 onward. Patients who remained on PBO were discontinued from the LTE after maintenance study unblinding. During the maintenance study, CS tapering was recommended for all patients receiving CS at maintenance baseline. At week 92 of the LTE, CS-free symptomatic remission rates and CS use were calculated using intention-to-treat analyses with a dose adjustment considered to be a treatment failure and with a dose adjustment not considered to be a treatment failure.
Results
Of the 284 patients in the randomised population who were treated with UST in the LTE, 139 were receiving CS at maintenance baseline. Of these, 92.8% (
Conclusion
UST maintenance therapy, with both q8wk and q12wk dosing regimens, was effective in reducing and eliminating the use of CS in patients with UC. Nearly all patients in the LTE who were in symptomatic remission at week 92, were not taking steroids.


