DOP78 Comparative study of the effectiveness of vedolizumab versus ustekinumab after anti-TNF failure (VERSUS-CD)
García García, M.J.(1);Rivero, M.(1);Fernández-Clotet, A.(2);de Francisco, R.(3);Sicilia, B.(4);Mesonero, F.(5);de Castro, M.L.(6);Casanova, M.J.(7);Bertoletti, F.(8);García Alonso, F.J.(9);López García, A.(10);Julián, B.(11);Calvet, X.(12);Barreiro-de Acosta, M.(13);Jara, L.(14);Varela, P.(15);Nuñez, A.(16);Ricart, E.(2);Riestra, S.(3);Arias, L.(4);Rodríguez, M.(17);Arranz, L.(18);Pajares, R.(19);Mena, R.(20);Calafat, M.(21);Camo, P.(22);Jiménez, L.(23);Ponferrada, A.(24);Madrigal, R.E.(25);Llao, J.(26);Sesé, E.(27);Almela, P.(28);Codesido, L.(29);de la Maza, S.(30);Leal, C.(31);Sánchez, E.(5);Pineda Mariño, J.R.(6);Domènech, E.(21);Chaparro, M.(7);P. Gisbert, J.(7);
(1)Hospital Universitario Marqués de Valdecilla- IDIVAL, Gastroenterology, Santander, Spain;(2)Hospital Clinic of Barcelona- CIBERehd, Gastroenterology, Barcelona, Spain;(3)Hospital Universitario Central de Asturias- Instituto de Investigación Sanitaria del Principado de Asturias ISPA, Gastroenterology, Oviedo, Spain;(4)Hospital Universitario de Burgos, IBD Unit- Gastroenterology, Burgos, Spain;(5)Hospital Universitario Ramón y Cajal, Gastroenterology, Madrid, Spain;(6)Hospital Álvaro Cunqueiro, Gastroenterology, Vigo, Spain;(7)Hospital Universitario de La Princesa- Instituto de Investigación Sanitaria Princesa IIS-IP- Universidad Autónoma de Madrid UAM- Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas CIBERehd, Gastroenterology, Madrid, Spain;(8)Hospital de la Santa Creu I Sant Pau, Gastroenterology, Barcelona, Spain;(9)Hospital Universitario Río Hortega, Gastroenterology, Valladolid, Spain;(10)Hospital del Mar, Gastroenterology, Barcelona, Spain;(11)Hospital Universitario Miguel Servet, Gastroenterology, Zaragoza, Spain;(12)Consorci Corporació Sanitària Parc Taulí, Gastroenterology, Sabadell, Spain;(13)Hospital Universitario Clínico de Santiago, Gastroenterology, Santiago de Compostela, Spain;(14)Hospital Universitario Fundación de Alcorcón, Gastroenterology, Alcorcón, Spain;(15)Hospital Universitario de Cabueñes, Gastroenterology, Gijón, Spain;(16)Hospital Universitario de Salamanca, Gastroenterology, Salamanca, Spain;(17)Hospital General Universitario de Ciudad Real, Gastroenterology, Ciudad Real, Spain;(18)Hospital Universitario Nuestra Señora de Candelaria, Gastroenterology, Tenerife, Spain;(19)Hospital Universitario Infanta Sofía, Gastroenterology, Madrid, Spain;(20)Consorci Sanitari de Terrasa, Gastroenterology, Barcelona, Spain;(21)Hospital Universitari Germans Trias I Pujol, Gastroenterology, Barcelona, Spain;(22)Hospital General San Jorge, Gastroenterology, Huesca, Spain;(23)Hospital Universitario de Fuenlabrada, Gastroenterology, Madrid, Spain;(24)Hospital Infanta Leonor, Gastroenterology, Madrid, Spain;(25)Hospital Clínico Universitario de Valladolid, Gastroenterology, Valladolid, Spain;(26)Althaia Xarxa Assistencial Universitària de Manresa, Gastroenterology, Manresa, Spain;(27)Hospital Universitario Arnau de Vilanova de Lleida, Gastroenterology, Lleida, Spain;(28)Hospital General Universitario de Castellón, Gastroenterology, Castellón, Spain;(29)Complexo Hospitalario Universitario de Ourense, Gastroenterology, Ourense, Spain;(30)Hospital Universitario de Basurto, Gastroenterology, Bilbao, Spain;(31)Consorci Hospitalari de Vic, Gastroenterology, Vic, Spain; GETECCU
Background
Main aim: To evaluate the retention rate of ustekinumab compared to vedolizumab in Crohn’s disease patients who failed anti-TNF therapy in clinical practice. Secondary aims: To compare the short-term and long-term effectiveness, and the safety of both treatments.
Methods
Results
755 patients were included (195 in the vedolizumab cohort and 560 in the ustekinumab cohort). After a median of 20 months (IQR 7.4-30) of follow-up, the survival rate for ustekinumab therapy was higher than vedolizumab (Figure 1). The propensity matching score verified the differences between both therapies. The short-term proportion of patients on clinical remission, steroid-free remission and clinical response was also superior in the ustekinumab cohort (Figure 2). In the long-term, significant differences were observed 2 years after the beginning of the treatments, although no differences in clinical response and remission rates were detected in patients who achieved clinical response at week 16 between both cohorts. Vedolizumab was discontinued in 142 patients and ustekinumab in 185, mainly due to primary non-response (52% in the vedolizumab and 58% in the ustekinumab cohort) and loss of response (34% and 25%, respectively) despite the fact that 35% of the patients required intensification. The predictive factors associated to the discontinuation of the therapy are described in table 1. Adverse events were observed, overall, in 12% of the patients, without differences between both groups (Table 2). Following the discontinuation of the treatment with vedolizumab/ustekinumab, other biologic agents were prescribed in 56% of the patients, and 27% underwent surgery.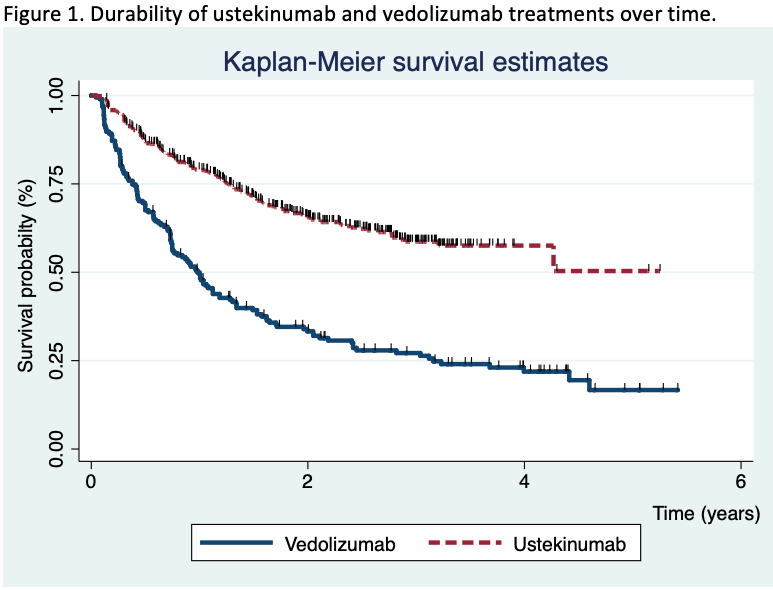



Conclusion
In clinical practice, a relatively high proportion of Crohn’s disease patients who received ustekinumab or vedolizumab for anti-TNF failure, maintained these drugs in the medium-long term, although ustekinumab retention rate was higher in comparison with vedolizumab.


