DOP80 Effectiveness of ustekinumab and vedolizumab in patients with Crohn’s disease refractory to anti-tumour necrosis factor: A multi-centre comparative study
H. Alric1, A. Amiot2, J. Kirchgesner3, X. Tréton4, M. Allez5, Y. Bouhnik4, L. Beaugerie3, F. Carbonnel1, A. Meyer1
1Hôpital Bicêtre, Service de gastro-entérologie, Le Kremlin-Bicêtre, France, 2Hôpital Henri Mondor, Service de gastro-entérologie, Créteil, France, 3Hôpital Saint-Antoine, Service de gastro-entérologie, Paris, France, 4Hôpital Beaujon, Service de gastro-entérologie et assistance nutritive, Clichy, France, 5Hôpital Saint-Louis, Service de gastro-entérologie, Paris, France
Background
There is no head-to-head trial comparing ustekinumab and vedolizumab in patients with Crohn’s disease (CD) refractory to anti-TNF. In France between May 2014 and November 2016, vedolizumab (and not ustekinumab) was reimbursed for patients who had failed anti-TNF. Then, between December 2016 and August 2018, ustekinumab (and not vedolizumab) was reimbursed for this category of patients. Since September 2018, both ustekinumab and vedolizumab are reimbursed in patients who are refractory to anti-TNF. The aim of this study was to compare effectiveness and safety of ustekinumab and vedolizumab in patients with CD refractory to anti-TNF.
Methods
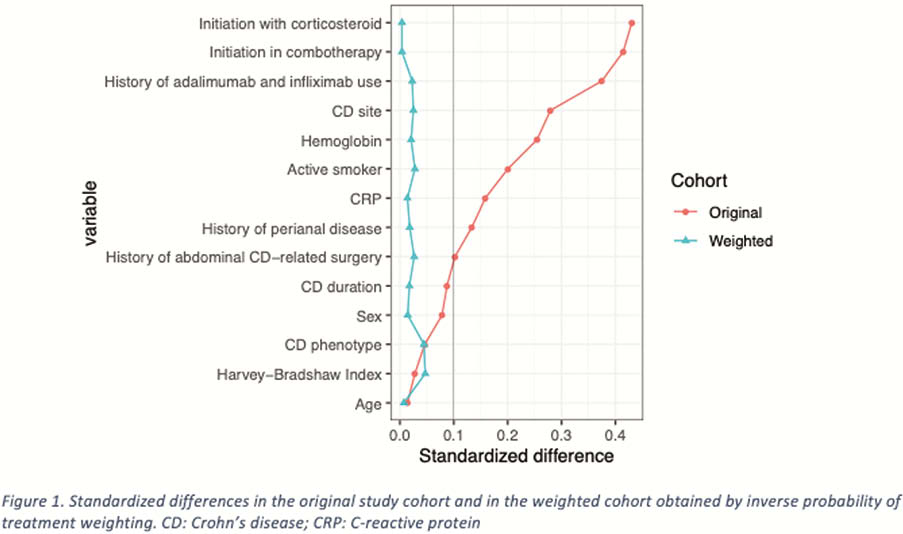
We studied all consecutive patients with active CD who were refractory to at least one anti-TNF, and were treated either with vedolizumab or ustekinumab, in five university hospitals of the Paris area, between May 2014 and August 2018. The primary endpoint was clinical remission rate at week 48. Adjustment according to propensity scores with inverse probability of treatment weighting was performed.
Results
239 patients were included, 107 received ustekinumab and 132 received vedolizumab. After propensity scoring with IPTW, there was no difference between the two groups (Figure 1). At week 48, the clinical remission rate was higher with ustekinumab than with vedolizumab (54.4% vs. 38.3%; OR =1.92, 95% CI [1.09–3.39]). At week 48, corticosteroid-free remission rate tended to be numerically higher with ustekinumab than with vedolizumab (44.7% vs. 34.0%; OR = 1.57, 95% CI [0.88–2.79]). Treatment persistence was significantly more frequent in the ustekinumab group (71.5% vs. 49.7%; OR = 2.54, 95% CI [1.40–4.62]). The dose optimisation rate at week 48 was higher with vedolizumab than with ustekinumab (53.5% vs. 30.1%; OR = 0.37, 95% Cl [0.21–0.67]). Subgroup analyses showed that ustekinumab was associated with higher clinical remission rates at week 48 in patients with ileal CD (OR = 3.49; 95% CI [1.33–9.17]), a penetrating phenotype (OR = 6.58; 95% CI [1.91–22.68]) and a history of perianal disease (OR = 2.48; 95% CI [1.04–5.93]). Regardless of treatment group, combotherapy was associated with a higher clinical remission rate at week 48 (OR = 1.93; 95% CI [1.09–3.43]).
Conclusion
This study suggests that, after 1 year of follow-up, ustekinumab is associated with a higher rate of clinical remission than vedolizumab in CD patients refractory to anti-TNF, particularly in those with ileal and penetrating disease.


