DOP81 Time to loss of efficacy among patients in remission following induction treatment with tofacitinib 10 mg BID who reduced tofacitinib dose or discontinued tofacitinib in OCTAVE sustain
M.C. Dubinsky1, J.W. Chou2, C. Su3, W. Wang3, I. Modesto4, X. Guo3, S. Vermeire5
1Icahn School of Medicine at Mount Sinai, New York, NY, USA, 2Division of Gastroenterology and Hepatology, Department of Internal Medicine, China Medical University Hospital, China Medical University, Taichung, Taiwan, Province of China, 3Pfizer Inc., Collegeville, PA, USA, 4Pfizer Inc., New York, NY, USA, 5Department of Gastroenterology, University Hospitals Leuven, Leuven, Belgium
Background
Tofacitinib is an oral, small-molecule JAK inhibitor for the treatment of ulcerative colitis (UC). Safety and efficacy of tofacitinib were evaluated in two Phase 3 induction studies (OCTAVE Induction 1 and 2; NCT01465763, NCT01458951), a 52-week, Phase 3 maintenance study (OCTAVE Sustain, NCT01458574) and an ongoing, open-label, long-term extension study (NCT01470612).1,2 Here, we assess time to treatment failure among patients in remission at the end of OCTAVE Induction 1 and 2 who enrolled in OCTAVE Sustain.
Methods
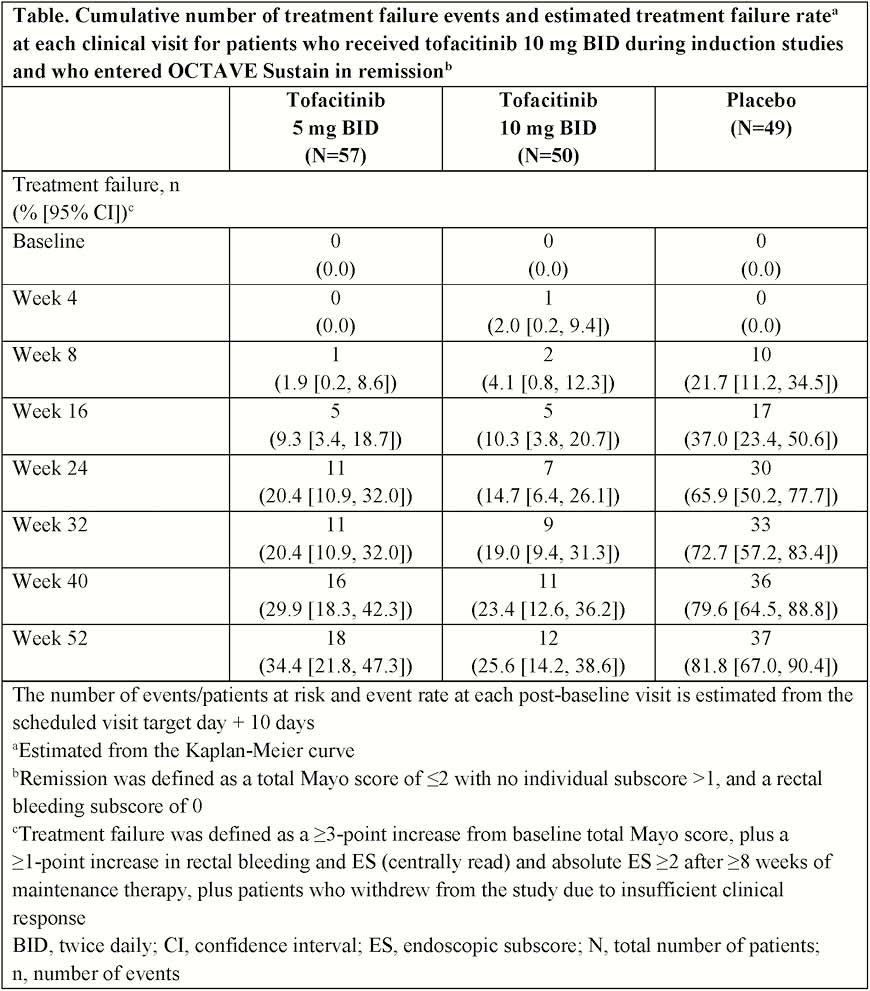
In OCTAVE Induction 1 and 2, patients received placebo or tofacitinib 10 mg twice daily (BID) for 8 weeks; clinical responders were re-randomised to placebo, tofacitinib 5 or 10 mg BID in OCTAVE Sustain for 52 weeks. Kaplan–Meier method was used to estimate median time to treatment failure (withdrawal due to insufficient clinical response) in patients who received 10 mg BID in induction studies and entered OCTAVE Sustain in remission (total Mayo score ≤2 with no subscore >1, and rectal bleeding subscore [RB] 0). Treatment failure: ≥3-point increase from baseline total Mayo score, plus ≥1-point increase in RB and endoscopic subscore (ES; centrally read) and absolute ES ≥2 after ≥8 weeks of maintenance therapy.
Results
Following induction, 156 patients in remission entered OCTAVE Sustain and received tofacitinib 5 mg BID (
Conclusion
For patients in remission following 8 weeks of induction treatment with tofacitinib 10 mg BID, median time to treatment failure for those who continued tofacitinib treatment (5 or 10 mg BID) was >52 weeks. After treatment interruption (remission patients who were re-randomised to placebo), median time to treatment failure was 169 days; this was similar to previously published time-to-treatment-failure data for patients with clinical response (including remission) following 8 weeks of induction treatment with tofacitinib who were re-randomised to placebo.3.
Sandborn WJ
Lichtenstein GR
Dubinsky MC


