DOP89 Infliximab and ustekinumab clearance during induction predicts post-induction endoscopic outcomes in patients with Crohn’s Disease
Wang, Z.(1);Kantasiripitak, W.(1);Verstockt, B.(2,3);João, S.(2,3);Ferrante, M.(2,3);Declerck, P.(1);D’Haens, G.(4);Laharie, D.(5);Vermeire, S.(2,3);Dreesen, E.(1);
(1)KU Leuven, Department of Pharmaceutical and Pharmacological Sciences, Leuven, Belgium;(2)University Hospitals Leuven, Department of Gastroenterology and Hepatology, Leuven, Belgium;(3)KU Leuven, Department of Chronic Diseases and Metabolism, Leuven, Belgium;(4)Amsterdam UMC, Department of Gastroenterology and Hepatology, Amsterdam, The Netherlands;(5)Université de Bordeaux, Department of Hepato-gastroenterology and Digestive Oncology, Bordeaux, France;
Background
Monitoring of monoclonal antibody clearance has been hypothesised to be an appealing approach for predicting treatment outcomes in patients with inflammatory bowel diseases. We aimed to investigate the benefits of monitoring infliximab and ustekinumab clearance in patients with Crohn’s disease (CD) based on data from clinical trials.
Methods
Data were obtained from patients with moderate-to-severe CD starting infliximab (n=108)1 or ustekinumab (n=80)2 therapy. Endoscopic remission (CD Endoscopic Index of Severity <3) and endoscopic response (≥50% decrease from baseline in simple endoscopic score for CD) were assessed at week (w)12 and w24 of infliximab and ustekinumab therapy, respectively. A priori prediction (based on covariate data only; at w0) and a posteriori prediction (Bayesian forecasting using measured drug concentrations; during treatment) were performed using previously built population pharmacokinetic models (NONMEM 7.5).3,4 Covariates of fecal calprotectin, albumin, CD activity index, and antibodies towards infliximab (ATIs) were used to estimate infliximab clearance. Albumin and body weight were used to estimate ustekinumab clearance.
Results
Patients achieving endoscopic remission at w12 had significantly lower infliximab clearance and higher infliximab serum concentration at w2 and w6 of treatment (P <0.05, Table 1). Patients achieving endoscopic response at w24 had significantly lower ustekinumab clearance at w4 and w8, as well as a significantly larger reduction in clearance relative to w0 (P <0.05, Table 1). However, ustekinumab serum concentrations at w4 and w8 were similar between patients with and without endoscopic response (P >0.2, Table 1).
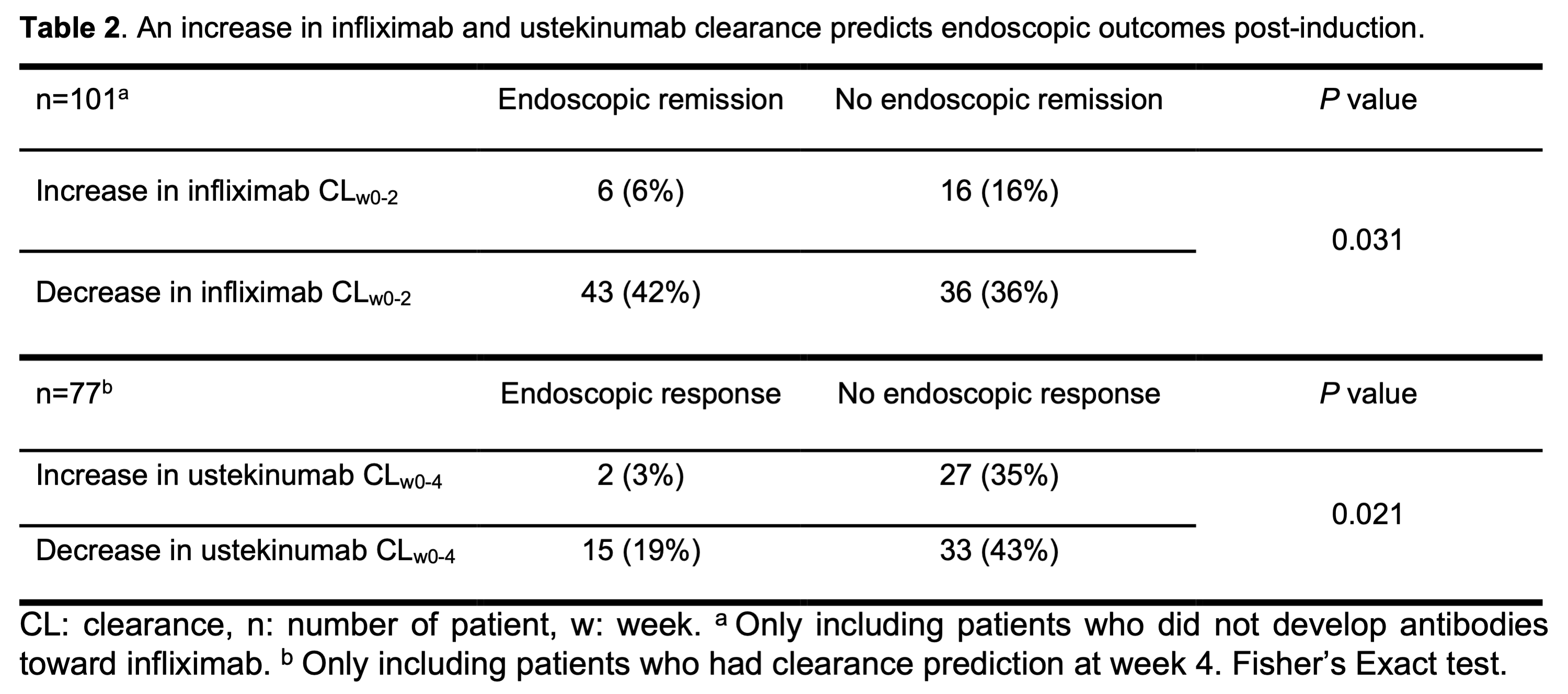
Most patients with an early increase in infliximab clearance (16/22; 73%) and ustekinumab clearance (27/29; 93%) did not reach the endoscopic endpoint (P <0.05; Table 2). However, a decrease in clearance was no guarantee for endoscopic remission during infliximab therapy (false predictive rate 46%) and response during ustekinumab therapy (false predictive rate 69%).
The infliximab clearance after start of induction therapy (at w2 and w6) was significantly higher in patients who developed ATIs during induction therapy (Figure 1).
Conclusion
Lower infliximab and ustekinumab clearance (absolute as well as relative to w0) early during induction predict more favourable endoscopic outcomes. In patients treated with ustekinumab, clearance monitoring may better predict endoscopic response at w24 as compared to standard therapeutic drug monitoring.
References
1. D'Haens et al.Gastroenterology 2018
2. Verstockt et al. J Crohns Colitis 2019.
3. Dreesen et al. Br J Clin Pharmacol 2021.
4. Wang et al. Br J Clin Pharmacol 2021.