OP01 Higher vs. standard adalimumab maintenance regimens in patients with moderately to severely active ulcerative colitis: Results from the SERENE-UC maintenance study
J.F. Colombel1, J. Panés2, G. D’Haens3, S. Schreiber4, R. Panaccione5, L. Peyrin-Biroulet6, E.V. Loftus Jr7, S. Danese8, E. Louis9, A. Armuzzi10, M. Ferrante11, H. Vogelsang12, J. Lefebvre13, T. Doan14, W. Xie15, B. Huang15, J. Petersson16, J. Kalabic17, A. Robinson18, W.J. Sandborn19
1Department of Gastroenterology, Icahn School of Medicine at Mt Sinai, New York, NY, USA, 2Department of Gastroenterology, Hospital Clinic Barcelona- IDIBAPS- CIBERehd, Barcelona, Spain, 3Department of Inflammatory Bowel Disease, Amsterdam, Amsterdam University Medical Centers, The Netherlands, 4Department of Internal Medicine, University Hospital Schleswig-Holstein, Kiel, Germany, 5Department of Medicine, University of Calgary, Calgary, Canada, 6Department of Gastroenterology, University Hospital of Nancy - Lorraine University, Vandoeuvre, France, 7Department of Internal Medicine, Mayo Clinic, Rochester, MN, USA, 8Department of Gastroenterology, Istituto Clinico Humanitas, Rozzano- Milan, Italy, 9Department of Gastroenterology, University Hospital CHU of Liège, Liège, Belgium, 10Department of Gastroenterology, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy, 11Department of Gastroenterology, University Hospitals Leuven - KU Leuven, Leuven, Belgium, 12Department of Gastroenterology, Medical University of Vienna, Vienna, Austria, 13Department of Clinical Studies, AbbVie Inc., North Chicago, IL, USA, 14Department of Pharmacovigillance, AbbVie Inc., North Chicago, IL, USA, 15Department of Statistics, AbbVie Inc., North Chicago, IL, USA, 16Department of Gastroenterology, AbbVie Inc., North Chicago, IL, USA, 17Department of Immunology, AbbVie Deutschland GmbH & Co. KG, Ludwigshafen, Germany, 18Department of Immunology, AbbVie Inc., North Chicago, IL, USA, 19Department of Gastroenterology, University of California at San Diego, La Jolla, CA, USA
Background
Adalimumab (ADA) is efficacious and well tolerated in adult patients with ulcerative colitis (UC).1,2 SERENE-UC (NCT02065622) is a study evaluating higher ADA dosing regimens from which induction study results have previously been reported3; here, we report data from the maintenance study.
Methods
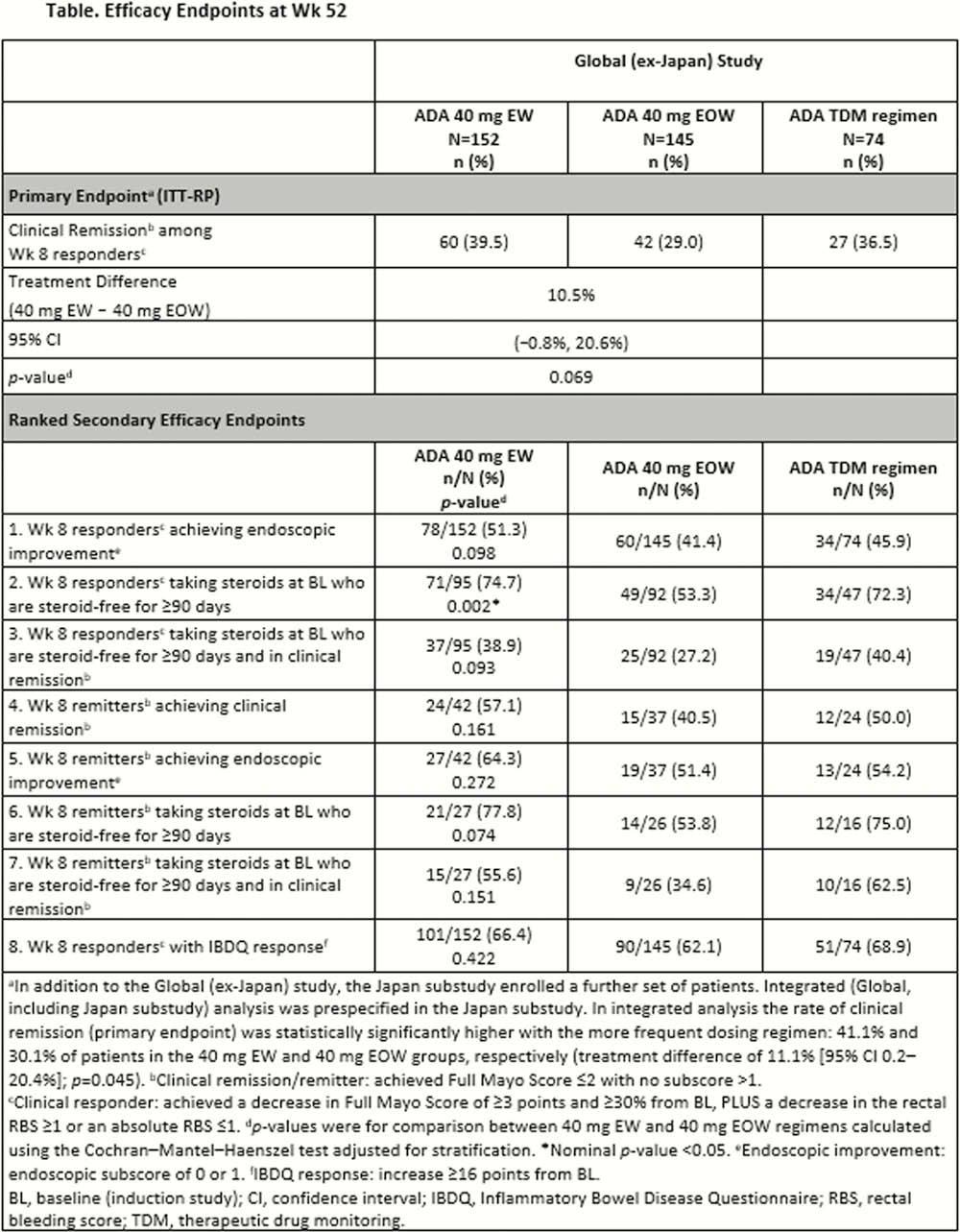
This Phase 3, double-blind, randomised, multicentre study evaluated higher vs. standard ADA dosing regimens for induction and maintenance therapy in adult patients with moderately to severely active UC. Following completion of the induction study at Week 8, all patients were re-randomised 2:2:1 to ADA 40 mg every week (40 EW), ADA 40 mg every other week (40 EOW), or exploratory ADA 40 mg with therapeutic drug monitoring (TDM) regimens, stratified by induction treatment regimen, clinical response status at Week 8, and clinical remission (CRem) status at Week 8 among Week 8 responders. Efficacy endpoints were evaluated in Week 8 responders (ITT-RP) unless indicated differently. The primary efficacy endpoint was proportion of patients achieving CRem (full Mayo Score ≤2 with no subscore >1) at Week 52. The endoscopic component of the Mayo Score was scored via central reading. Non-responder imputation was used for missing values. Comparisons between 40 EW and 40 EOW dosing regimens used the Cochran–Mantel–Haenszel test adjusted for stratification. Safety assessment included a collection of adverse events (AEs), vital signs, and laboratory data.
Results
Of 852 patients in the Global (ex-Japan) induction study, 757 were re-randomised at Week 8 and 371 were included in the ITT-RP. In the ITT-RP, induction baseline demographics and disease characteristics were generally balanced across the 3 treatment arms; range across arms for mean UC disease duration was 6.2–7.8 years, 6.8–11.7% had prior infliximab use, and 62.5–63.5% were receiving corticosteroids. The overall mean ADA exposure was 252.2 days. The table displays results for efficacy endpoints. The observed safety profile was similar between maintenance treatment arms, including AEs of special interest (generally <1% across arms).
Conclusion
In the Global (ex-Japan) study of SERENE-UC, CRem at Week 52 was numerically higher among Week 8 responders in patients receiving ADA 40 EW compared with 40 EOW maintenance regimens (∆ = 10.5%), but not statistically significant. Higher maintenance treatment with 40 EW was generally safe and well tolerated with a similar safety profile to 40 EOW. No new long-term safety concerns were observed.
Reinisch W,
Sandborn WJ,
Panés J,


