OP04 Safety analysis of filgotinib for Ulcerative Colitis: Results from the phase 2b/3 SELECTION study and phase 3 SELECTIONLTE long-term extension study
Schreiber, S.W.(1);Watanabe, M.(2);Yun, C.(3);Zhou, Y.(3);Zhao, S.(3);Hsieh, J.(3);Moerch, U.(4);Rogler, G.(5);Loftus Jr, E.V.(6)
(1)Kiel University, Department of Medicine I, Kiel, Germany;(2)Tokyo Medical and Dental University, TMDU Advanced Research Institute, Tokyo, Japan;(3)Gilead Sciences, Inc., Foster City, United States;(4)Gilead Sciences, Inc., Copenhagen, Denmark;(5)University Hospital of Zurich- University of Zurich, Clinic for Gastroenterology and Hepatology, Zurich, Switzerland;(6)Mayo Clinic, Department of Internal Medicine- Division of Gastroenterology and Hepatology, Rochester- MN, United States
Background
Filgotinib (FIL) is an oral preferential Janus kinase (JAK) 1 inhibitor in development for the treatment of inflammatory diseases. FIL for the treatment of moderately to severely active ulcerative colitis (UC) was evaluated in the phase 2b/3, double-blind, placebo (PBO)-controlled SELECTION study (NCT02914522) and its long-term extension (LTE) study (NCT02914535). Here we report safety results from the FIL UC program.
Methods
Patients received FIL 100 mg, FIL 200 mg or PBO (2:2:1) once daily orally for up to 11 weeks for induction (cohort 1). At week 11, FIL induction responders were rerandomized 2:1 to continue FIL or receive PBO maintenance for 47 weeks (cohort 2). Week 10 non-responders and patients with worsening disease during the maintenance study were eligible for open-label FIL in the LTE. Patients completing the maintenance study could continue blinded dosing in the LTE. Cohort 3 comprised cohorts 1 and 2 and the LTE. Exposure-adjusted incidence rates (EAIRs) and exposure-adjusted event rates (EAERs) per 100 patient-years (PYs) were calculated for treatment-emergent adverse events (AEs) by treatment group in cohorts 1 and 2 (EAIR) and cohort 3 (EAER).
Results
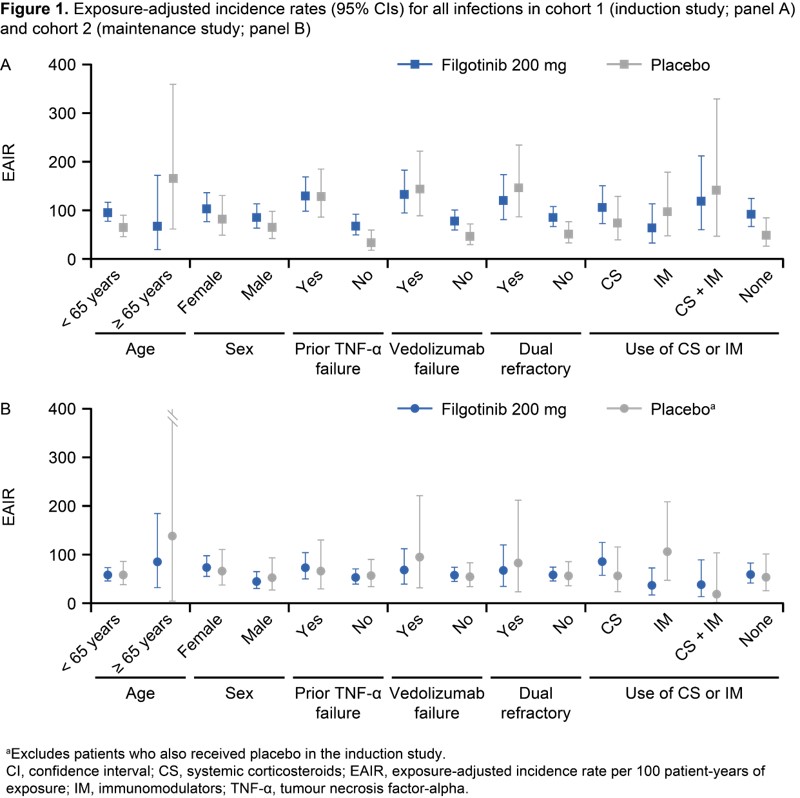
In cohort 1, 1069 patients received FIL and 279 patients received PBO; baseline characteristics were generally similar across treatment groups (overall mean age, 43 years; mean UC duration, 8.4 years; mean Mayo Clinic Score, 9.0). EAIRs for AEs of interest were similar across treatment groups in cohorts 1 and 2 (Table 1). Treatment exposure for PBO, FIL 100 mg or FIL 200 mg in cohort 3 (i.e. cohorts 1 + 2 + the LTE) was 318, 360 and 1207 PYs, and median treatment duration was 12, 11 and 67 weeks, respectively. One case of pulmonary embolism occurred with FIL 200 mg induction and three venous thrombosis cases occurred with PBO maintenance/LTE (cohort 3) (Table 2). EAERs for all infections were similar across treatment groups, the most common being nasopharyngitis (Table 2). Opportunistic infections were rare. EAERs for serious infections were low across treatment groups (2.2 [PBO], 3.5 [FIL 100 mg], 2.2 [FIL 200 mg]), the most common being appendicitis (Table 2). EAERs for herpes zoster (HZ) were low in all treatment groups (0.3 [PBO], 0.3 [FIL 100 mg], 1.8 [FIL 200 mg]) (Table 2). HZ infections were cutaneous only and only one was serious. EAIRs for all infections in cohorts 1 and 2 were generally numerically higher for both PBO and FIL in patients over (vs under) 65 years old and in those with (vs without) biologic treatment failure (Figure 1).

Conclusion
FIL was well tolerated in patients with UC. Aggregation of AEs typical for pan-JAK inhibition was not observed, consistent with preferential JAK-1 inhibition with FIL.


