OP05 Outcome of induction therapy with vedolizumab in children: Results from the prospective, multi-centre VEDOKIDS study
Shavit, Z.(1);Stein, R.(2);Aloi, M.(3);Ledder, O.(4);Focht, G.(4);Urlep, D.(5);Hyams, J.(6);Broide, E.(7);Vais, B.(8);Levine, J.(9);Shouval , D.(10);Matar, M.(10);Assa, A.(10);Rosh, J.(11);Hussey, S.(12);Markowitz, J.(13);Yerushalmy-Feler, A.(14);Miele, E.(15);Shaoul, R.(16);Russell, R.K.(17);Turner, D.(4);
(1)Shaare Zedek Medical Center, The Pediatric Gastroenterology and Nutrition Institute, Jerusalem, Israel;(2)Children’s Hospital of Philadelphia, Center for Pediatric Inflammatory Bowel Disease, Philadelphia, United States;(3)Sapienza University of Rome- Umberto I Hospital, Pediatric Gastroenterology and Liver Unit, Rome, Italy;(4)Shaare Zedek Medical Center- Jerusalem, The Juliet Keidan Institute of Paediatric Gastroenterology and Nutrition, Jerusalem, Israel;(5)University Children’s Hospital- Ljubljana, Pediatric Gastroenterology, Ljubljana, Slovenia;(6)Connecticut Children's Medical Center, Center for Digestive Diseases, Connecticut, United States;(7)Shamir Medical Center, Paediatric Gastroenterology and Nutrition, Beer Yaakov, Israel;(8)Sheba Medical Center, Pediatric Gastroenterology, Ramat Gan, Israel;(9)NYU Langone Health, Pediatric Gastroenterology, New York, United States;(10)Schneider Children’s Medical Center, The Institute of Gastroenterology- Nutrition and Liver Diseases, Petah Tikva, Israel;(11)Goryeb Children's Hospital/Atlantic Health, Pediatric Gastroenterology, New Jersey, United States;(12)Children’s Health Ireland- Crumlin, Pediatric Gastroenterology, Dublin, Ireland;(13)The Feinstein Institute for Medical Research, Pediatric Gastroenterology, Northwell, United States;(14)Tel Aviv Sourasky Medical Center, Pediatric Gastroenterology- Liver and Nutrition, Tel Aviv, Israel;(15)University of Naples Frederico II, Pediatric Gastroenterology, Naples, Italy;(16)Rambam Medical Center, Pediatric Gastroenterology Unit, Haifa, Israel;(17)The Royal Hospital for Children & Young People, Pediatric Gastroenterology and Nutrition, Edinburgh, United Kingdom;
Background
Limited data are available on the use of Vedolizumab (VDZ) in paediatric Crohn’s Disease (CD) and Ulcerative Colitis (UC). We evaluated the effectiveness and safety of VDZ to induce remission at week 14 in the prospective, multicenter VEDOKIDS study.
Methods
We enrolled children (age 0-18 years) with CD or UC commenced on VDZ with a standardized dosing of 177mg/BSA up to 300mg at 0, 2, 6 and q8 weeks thereafter. Non-responders had their dose escalated to q4wks at the discretion of the local physician. Explicit demographic, clinical and safety data were prospectively recorded via REDcap. Clinical remission was defined as steroid- and EEN-free remission (i.e. wPCDAI<12.5 or PUCAI<10) without the need for new medications. Complete remission was defined as clinical remission with normal CRP and ESR. Predictors of response were explored by Logistic regression.
Results
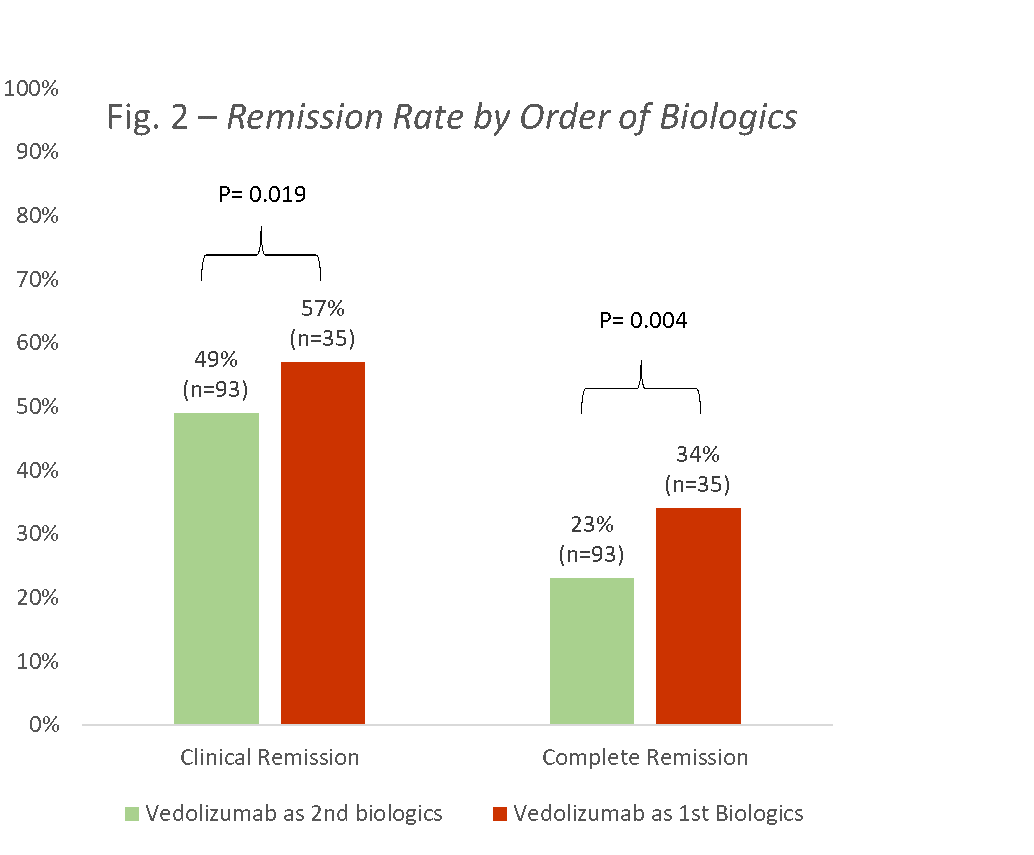
128 children were enrolled, 60 (47%) with CD, and 68 (53%) with UC (58 (45%) males, mean age 13.8±3.6, 93 (73%) failed previous anti-TNF, median disease duration 2.3 years (IQR 0.9-4.7)). Using the ITT principle, clinical and complete remission rates for CD at week 14 were 30% and 20%, respectively, and for UC 50% and 38%, respectively (Fig 1). Clinical remission rates of those receiving VDZ as first line biologics versus second line were 57% and 34%, respectively (p=0.019; Fig 2); the corresponding complete remission rates were 49% and 23% (p=0.004).
In the UC group, disease activity at baseline measured by the PUCAI predicted clinical remission at week 14 (OR=0.95, 95%CI 0.93-0.98; median baseline PUCAI 15 (IQR 0-30) in those achieving remission and 45 (20-55) in those who did not; p=0.002). ESR (OR=0.94, 95%CI 0.89-0.98; p=0.009) and a trend towards extensive disease (L3 vs. L1 and L2; OR 0.14, 95%CI 0.18-1.036, p=0.054) predicted clinical remission in CD.
During the 14 weeks, 113 adverse events (AE) were recorded in 58 children: 28 AEs were possibly related to VDZ, all of which were mild-moderate and only 3 (11%) led to discontinuation of VDZ (leukocytoclastic vasculitis, myalgia and dyspnea). There were 18 serious AEs, only one was graded as possibly related to VDZ (headache). There were 18 non-serious cases (19%) of upper respiratory infections (pharyngitis, tonsilitis, parotitis, and otitis media) and one Campylobacter jejuni which was graded as serious.

Conclusion
In this prospective multicenter study, VDZ was safe and effective for inducing remission in a refractory cohort of paediatric IBD, more so in UC. Disease severity and extent at baseline may predict clinical response.


