OP09 Proactive Therapeutic Drug Monitoring is superior to standard treatment during maintenance therapy with infliximab; results from a 52-week multicentre randomised trial of 450 patients; the NOR-DRUM B study
Jørgensen, K.K.(1);Syversen, S.W.(2);Goll, G.L.(2); Bjørlykke, K.H.(1,3);Sandanger, Ø.(4);Sexton, J.(2);Brun, M.K.(2,3);Noraberg, G.(5);Ystrøm, C.M.(6); Seeberg, K.A.(7);Blomgren, I.M.(8);Torp, R.(9);Mørk, C.(10);Kvien, T.K.(2,3); Bolstad , N.(4);Haavardsholm, E.A.(2,3);Jahnsen, J.(1,3);
(1)Akershus University Hospital, Dept. of Gastroenterology, Oslo, Norway;(2)Diakonhjemmet Hospital, Division of Rheumatology and Research, Oslo, Norway;(3)University of Oslo, Faculty of Medicine, Oslo, Norway;(4)Oslo University Hospital, Section of Dermatology, Oslo, Norway;(5)Hospital of Southern Norway Trust, Dept. of Gastroenterology, Arendal, Norway;(6)Innlandet Hospital Trust, Dept. of Medicine, Elverum, Norway;(7)Vestfold Hospital Trust, Dept. of Gastroenterology, Tønsberg, Norway;(8)Fonna Hospital Trust, Dept. of Gastroenterology, Haugesund, Norway;(9)Innlandet Hospital Trust, Dept. of Medicine, Hamar, Norway;(10)Akershus Dermatology Center, Dept. of Dermatology, Lørenskog, Norway; The NOR-DRUM study group
Background
Proactive therapeutic drug monitoring (TDM), individualized treatment based on scheduled assessments of serum drug levels, has been proposed to optimize efficacy and safety of infliximab and other biologic drugs. However, it is unclear whether this strategy improves clinical outcomes.
Methods
In this 52-week randomised, open-label, multicenter trial, adult patients with an established diagnosis of ulcerative colitis (UC), Crohn’s disease (CD), rheumatoid arthritis (RA), spondyloarthritis (SpA), psoriatic arthritis (PsA), and psoriasis (Ps) receiving infliximab therapy for a minimum of 30 weeks were randomly assigned to proactive TDM or standard infliximab treatment. In the TDM group, infliximab dosage was adjusted according to an algorithm designed to maintain serum infliximab levels within the therapeutic range 3-8 mg/L. In the standard treatment group, infliximab dosage was based on clinical judgement.The primary endpoint was sustained disease control during the 52 week study period.
Results
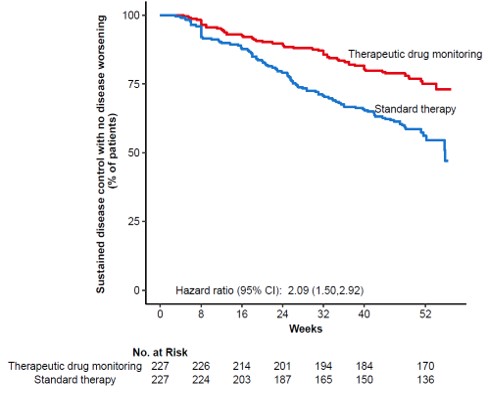
In total, 458 patients were randomised of whom 454 (UC 81, CD 66, RA 79, PsA 53, SpA 138, Ps 37) received the allocated strategy and were included in the primary analyses. The two groups were balanced regarding baseline demographics, clinical and treatment characteristics. Sustained disease control without disease worsening was observed in 167 (73.6 %) patients in the TDM group and in 127 (55.9%) patients in the standard treatment group. The estimated adjusted difference was 17.6% (95% confidence interval (CI), 9.0-26.2, p<0.001), favouring TDM (figure 1). Results were consistent in sensitivity analyses. Time to disease worsening was shorter in the standard treatment group, hazard ratio 2.1 (95% CI 1.5-2.9) (figure 2). Other secondary endpoints reflecting change in disease activity and patient reported outcomes from baseline to week 52 did not show significant differences between the two groups. During the trial, the mean infliximab dose (4.8 mg/kg) and median serum level of infliximab (5.8 mg/L) were comparable in both groups. Twenty-one (9%) patients in the TDM group and 27 (15%) in the standard treatment group developed clinically significant levels of anti-drug antibodies (≥50µg/L). Adverse events were reported in 137 (60%) and 142 (63%) patients in the TDM and standard treatment groups, respectively.
Figure 1

Figure 2
Conclusion
This large randomised controlled trial demonstrates that proactive TDM is superior to standard treatment for maintaining disease control without disease worsening in patients on maintenance therapy with infliximab. These results support implementation of proactive TDM as a general strategy during maintenance therapy with infliximab and have the potential to change clinical practice across specialities.


