OP17 Maintenance phase propensity score adjusted effectiveness and persistence at week-52 in biologic-naïve Ulcerative Colitis patients treated with vedolizumab or anti-TNF (VEDO IBD-study)
Plachta-Danielzik, S.(1);di Giuseppe, R.(2);Bokemeyer, B.(3);Efken, P.(4);Mohl, W.(5);Krause, T.(6);Hoffstadt, M.(7);Ehehalt, R.(8);Trentmann, L.(9);Schweitzer, A.(10);Jessen, P.(11);Franzenburg, S.(1);Hartmann, P.(4);Schreiber, S.(12);
(1)Kompetenznetz Darmerkrankungen, Studienabteilung, Kiel, Germany;(2)Kompetenznetz Darmerkrankungen, Statistics and Biometry, Kiel, Germany;(3)Interdisciplinary Crohn Colitis Centre Minden, Crohn Colitis Centre, Minden, Germany;(4)Gastroenterology Practice, Gastroenterology, Minden, Germany;(5)Gastroenterology Practice, Gastroenterology, Saarbrücken, Germany;(6)Gastroenterology Practice, Gastroenterology, Kassel, Germany;(7)Gastroenterology Practice, Gastroenterology, Iserlohn, Germany;(8)Gastroenterology Practice, Gastroenterology, Heidelberg, Germany;(9)Gastroenterology Practice, Gastroenterology, Bremen, Germany;(10)Gastroenterology Practice, Gastroenterology, Münster, Germany;(11)Gemeinschaftspraxis im Medicum, Gastroenterology, Altenholz, Germany;(12)Clinic of General Internal Medicine I, University Hospital Schleswig-Holstein- Campus Kiel, Kiel, Germany;
Background
In this real-world-evidence (RWE) study we aimed to analyse the persistence of biologic therapy in biologic-naïve ulcerative colitis (UC) patients and to compare 1-year effectiveness of vedolizumab (VDZ) and anti-TNF.
Methods
Between 2017 and 2020, 1200 consecutively enrolled biologic-naïve and biologic- experienced patients with UC and Crohn´s disease (CD) were prospectively included in the VEDOIBD-Registry from 45 IBD-experienced centres across Germany. After exclusion of bio-experienced patients, CD and missing outcomes, the final sample consisted of 274 biologic-naïve UC-patients with 1-year follow-up data. Switchers of a drug were considered as treatment failure (modified intention-to-treat analysis; mITT) while switchers were excluded from per protocol analysis (PP). Clinical response modified (reduction of partial Mayo score (pMayo) from baseline to 1-year by >3 points or a reduction of at least 30% compared to baseline or reaching remission at 1-year) and (steroid-free) remission rates (pMayo ≤1 plus a bleeding subscore=0 (and no systemic use of steroids or budesonide at 1-year)) were predefined as outcomes. To reduce the effect of confounders, PS adjustment with inverse probability of treatment weighting (IPTW) was implemented. A weighted logistic regression was used, and the results were reported as odds ratio (OR) and 95% confidence interval (CI).
Results
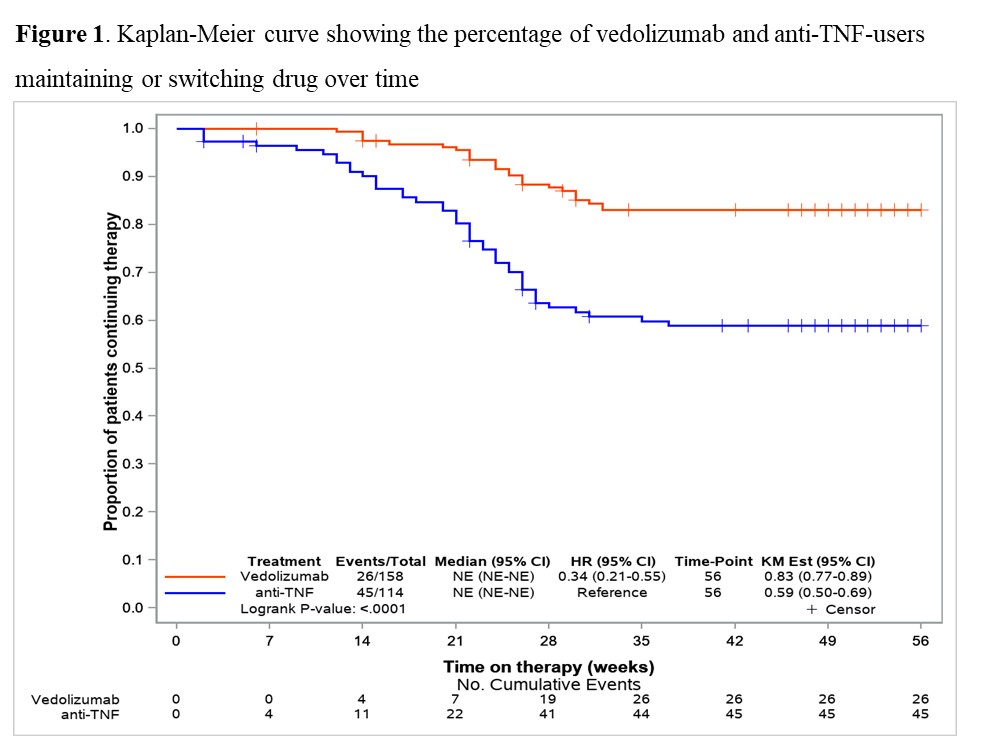
158 VDZ and 116 anti-TNF (ADA: 27.6%, IFX: 57.8%, GOL: 14.7%) biologic-naïve UC-patients were included in this prospective RWE comparing the effectiveness of VDZ vs anti-TNF. Until week 52 significantly more patients switched to another biologic-drug in the anti-TNF group than in the VDZ group (40.5% vs 16.5%; p<0.001) (Fig. 1). In mITT, clinical response at 1-year was significantly higher in VDZ than in anti-TNF treated patients (61.7% vs. 40.3%; OR 2.39 (95% CI 1.39-4.10)). VDZ also tended to be superior to anti-TNF for (steroid-free) remission (Tab. 1; p=0.058 (p=0.051)). In the PP-analysis, VDZ showed numerically higher 1-year effectiveness, but this did not reach statistical significance (Tab. 1). Analysing week-14 induction phase responders (Tab. 2), VDZ had numerically higher effectiveness rates compared to anti-TNF but without significant difference.
Conclusion
The 1-year maintenance findings suggested, in line with our previous induction phase data, only moderate long-term effectiveness in both groups. However, besides the significant response data, VDZ showed numerically higher remission rates compared to anti-TNF though only borderline significant. The higher treatment persistence of VDZ vs anti-TNF, along with the higher effectiveness, may suggest VDZ as a first-line biologic therapy option in UC patients.