OP20 The gut microbiota during biological therapy for inflammatory bowel disease
C. Caenepeel1, S. Viera-Silva2, B. Verstockt3, K. Machiels3, N.A. Davani3, J. Sabino3,4, M. Ferrante3,4, J. Raes2, S. Vermeire3,4
1Department of Chronic Diseases, Metabolism and Ageing, KU Leuven, Kessel-Lo, Belgium, 2Department of Microbiology and Immunology, Rega Institute for Medical Research, Leuven, Belgium, 3Department of Chronic Diseases, Metabolism and Ageing, KU Leuven, Leuven, Belgium, 4Department of Gastroenterology and Hepatology, University Hospitals Leuven, Leuven, Belgium
Background
The expansion of therapeutic options in IBD brought forward a need to personalise treatment. Gut inflammation in inflammatory bowel disease (IBD) patients has been associated with reduced microbial richness and abundance of SCFA producers. We aimed to explore the longitudinal impact of treatment on the inflammatory burden and faecal microbiota in patients with CD and UC, treated with anti-tumour necrosis factor (anti-TNF) therapy, vedolizumab (VDZ) or ustekinumab (UST).
Methods
We analysed faecal samples from 349 IBD patients (112 UC, 237 CD) initiating biological therapy (anti-TNF; VDZ; UST), regardless baseline disease activity, between 2010 and 2019 at a tertiary referral centre. Samples were collected at week 0, 14 and 24. Microbiota phylogenetic profiling was conducted by 16S rRNA gene sequencing and faecal cell counts were determined using flow cytometry. Moisture levels were measured using lyophilisation. Disease activity and response were assessed by faecal calprotectin (FCal). Statistics were performed in R, v3.5.1. Enterotyping was based on the Dirichlet multinomial mixtures approach.
Results
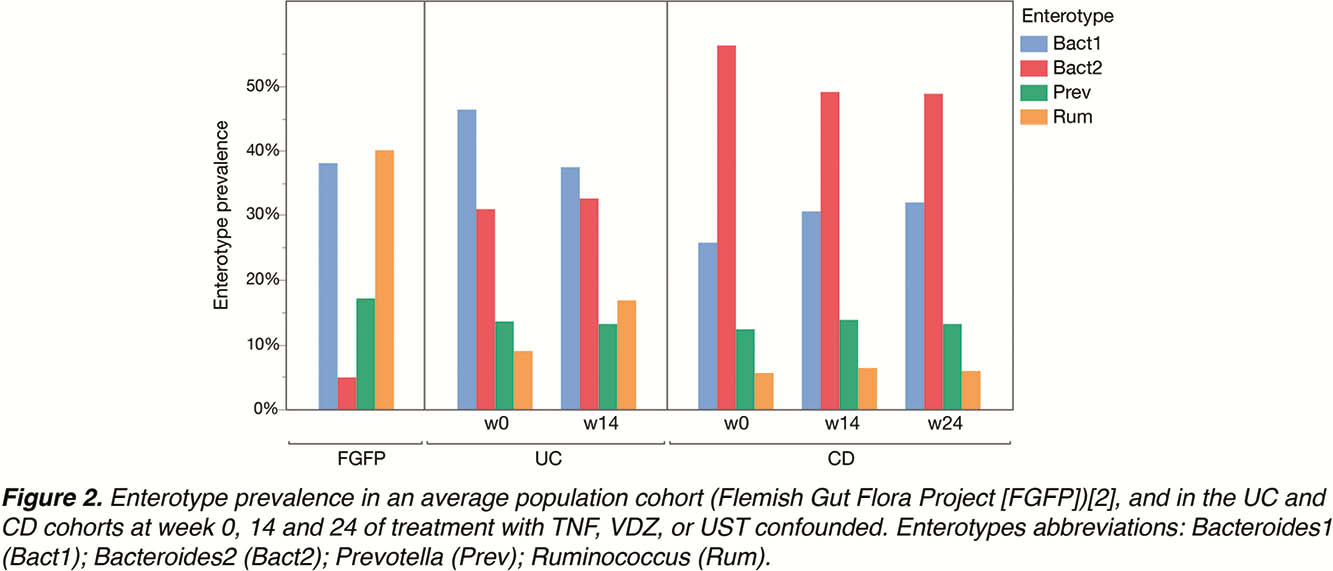
Faecal microbiota profiles showed high diversity, with samples classified into all four enterotypes (
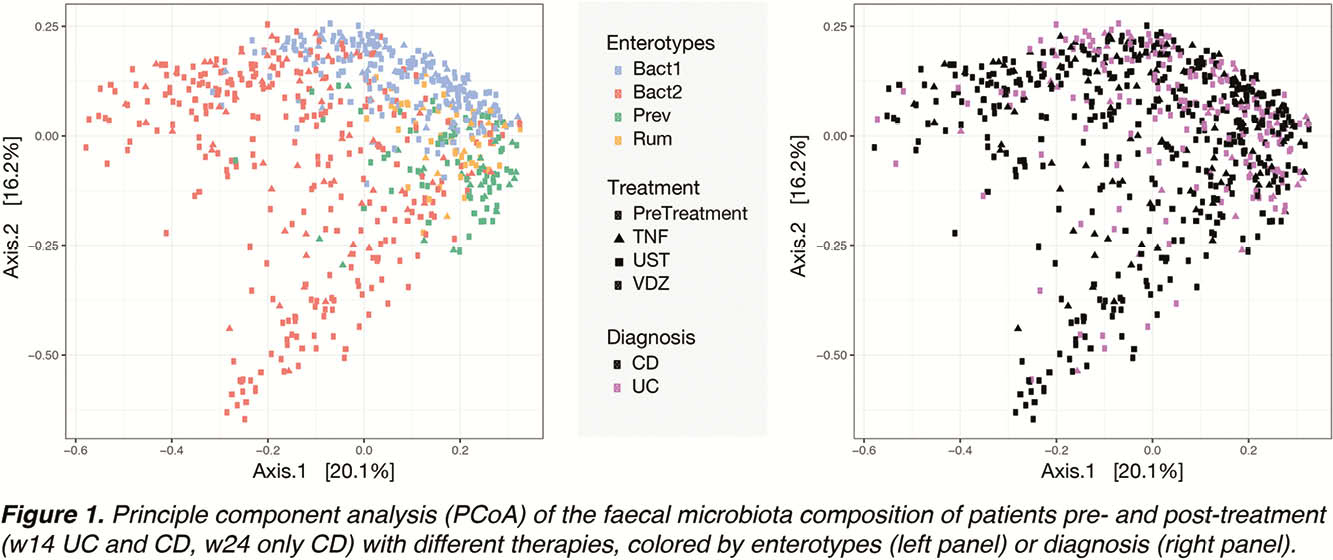
A slight non-significant decrease in the Bact2 enterotype prevalence was observed in the CD, but not in the UC cohort, during treatment.
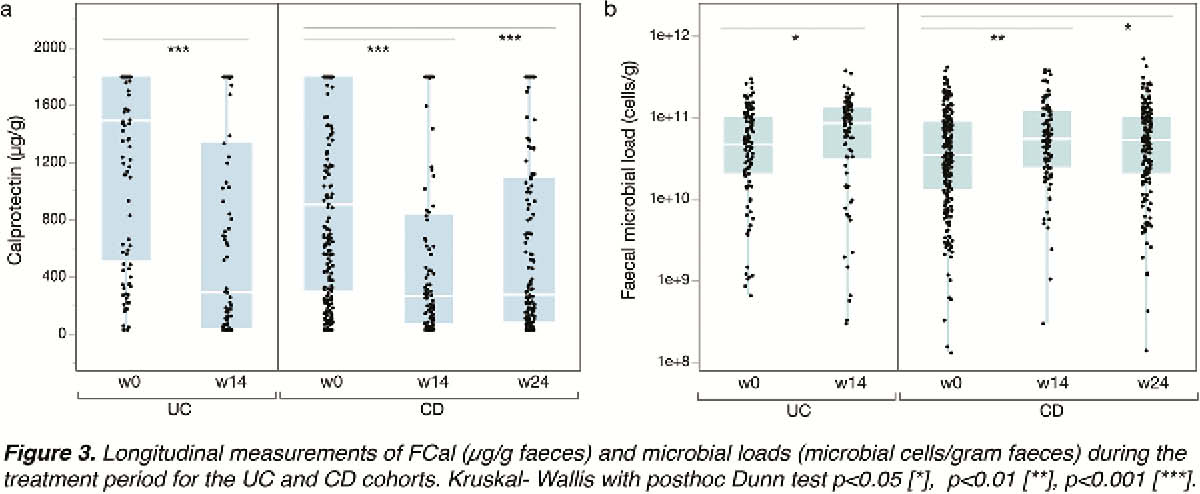
A significant decrease in FCal concentrations (w0 vs. 14
Only treatment-associated variables—week of treatment (
Conclusion
The prevalence of the inflammatory Bact2 enterotype was 5- to 10-fold higher in CD and UC patients as compared with controls. Although initiation of biological therapies leads to a decrease in inflammation levels as witnessed by faecal calprotectin and increase in microbial richness, a shift in enterotypes did not occur.


