OP23 The efficacy and safety of guselkumab induction therapy in patients with moderately to severely active Ulcerative Colitis: Phase 2b QUASAR Study results through week 12
Dignass, A.(1);Rubin, D.(2);Bressler, B.(3);Huang, K.H.(4);Shipitofsky, N.(4);Germinaro, M.(4);Zhang, H.(4);Johanns, J.(4);Feagan, B.(5);Sandborn, W.(6);Sands, B.(7);Hisamatsu, T.(8);Lichtenstein, G.(9);Panes, J.(10);Allegretti , J.(11);
(1)Agaplesion Markus Hospital, Department of Medicine, Frankfurt, Germany;(2)The University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, United States;(3)University of British Columbia, Division of Gastroenterology, Vancouver, Canada;(4)Janssen Research & Development- LLC, Immunology, Spring House, United States;(5)Western University, Robarts Clinical Trials, Ontario, Canada;(6)University of California San Diego, Division of Gastroenterology, La Jolla, United States;(7)Icahn School of Medicine at Mount Sinai, Dr. Henry D. Janowitz Division of Gastroenterology, New York, United States;(8)Kyorin University, Department of Gastroenterology and Hepatology, Tokyo, Japan;(9)University of Pennsylvania, Department of Medicine- Division of Gastroenterology, Philadelphia, United States;(10)Hospital Clínic de Barcelona, IDIBAPS- CIBERehd, Barcelona, Spain;(11)Brigham and Women’s Hospital Crohn’s and Colitis Center, Division of Gastroenterology- Hepatology and Endoscopy, Boston, United States; QUASAR Investigators
Background
The QUASAR Induction Study 1 (NCT04033445) is a phase 2b randomized, double-blind, placebo-controlled, dose-ranging study to evaluate the efficacy and safety of guselkumab (GUS), an interleukin-23 p19 subunit antagonist, as induction therapy in patients with moderately to severely active ulcerative colitis (UC) who had an inadequate response or intolerance to conventional (ie, thiopurines or corticosteroids) or advanced therapy (ie, tumor necrosis factor alpha antagonists, vedolizumab, or tofacitinib).
Methods
Patients included in these analyses had moderately to severely active UC (defined as a modified Mayo score of 5 to 9, inclusive) with a Mayo rectal bleeding subscore ≥ 1 and a Mayo endoscopy subscore ≥ 2 obtained during central review of video endoscopy at baseline. Patients were randomized 1:1:1 to receive IV GUS 200 mg, 400 mg, or placebo at Weeks 0, 4, and 8. The primary endpoint was clinical response at Week 12, and major secondary endpoints included clinical remission, symptomatic remission, endoscopic improvement, histo-endoscopic mucosal improvement, and endoscopic normalization at Week 12. Type 1 error was controlled at the 0.05 significance level for the primary endpoint; no other endpoints were controlled for multiplicity. Safety was assessed through Week 12.
Results
Three hundred thirteen patients were randomized in the primary analysis population (mean age, 41.6 yrs; male 59.1%, mean UC duration, 7.55 yrs; mean Mayo score, 9.2; endoscopy subscore of 3 indicating severe disease, 70%; baseline oral corticosteroid use, 39.6%). Approximately 50% had a prior inadequate response or intolerance to advanced therapy for UC. The baseline demographics and disease characteristics were generally similar among treatment groups (Table 1). At Week 12, a significantly greater proportion of patients treated with GUS 200 mg and 400 mg achieved clinical response compared with placebo (61.4% and 60.7% vs 27.6%, respectively, both p<0.001). A greater proportion of GUS-treated patients compared with placebo-treated patients achieved the major secondary endpoints at Week 12 (Figure 1). The proportions of patients reporting adverse events, serious adverse events, and adverse events leading to discontinuation in the GUS groups were not greater compared with placebo (Table 2). No serious infections were reported for GUS. No cases of malignancy or death were reported.

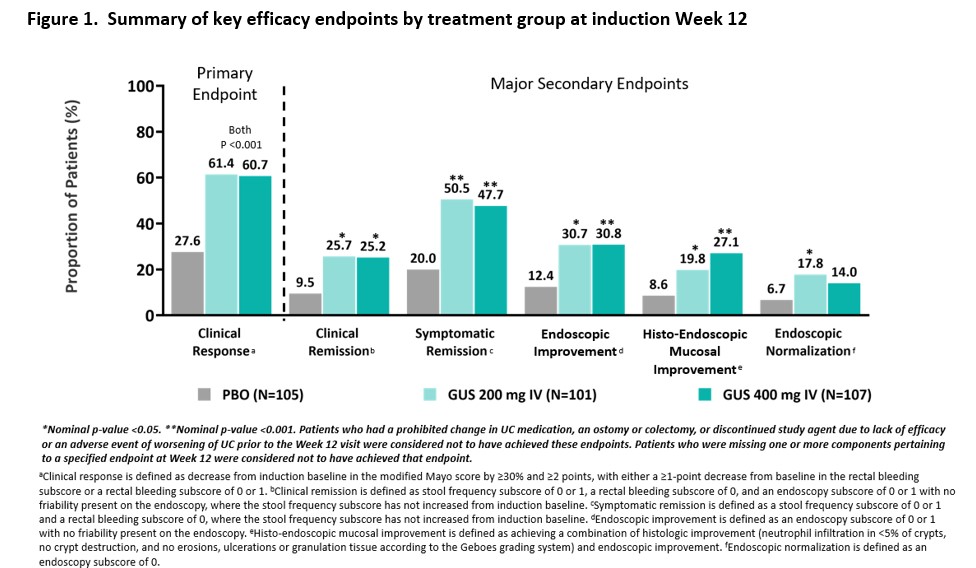

Conclusion
In patients with moderately to severely active UC, GUS induction treatment demonstrated superior efficacy compared with placebo treatment. Overall, safety results through Week 12 were consistent with the known safety profile of GUS in approved indications. The efficacy and safety of GUS 200 mg and 400 mg were comparable.


