OP27 Long-term safety and efficacy of risankizumab treatment in patients with Crohn’s disease: Final results from the Phase 2 open-label extension study
M. Ferrante1, B.G. Feagan2, J. Panés3, F. Baert4, E. Louis5, O. Dewit6, A. Kaser7, W.R. Duan8, D. Gustafson9, X. Liao10, K. Wallace9, J. Kalabic11, G.R. D’Haens12
1Department of Gastroenterology and Hepatology, University Hospitals Leuven, Leuven, Belgium, 2Clinical Trials Design and Management, University of Western Ontario, London, Canada, 3Department of Inflammatory Disease, Hospital Clinic Barcelona, Barcelona, Spain, 4Department of Gastroenterology, AZ Delta, Roeselare-Menen, Belgium, 5Department of Gastroenterology, University of Liège and CHU, Liège, Belgium, 6Department of Gastroenterology, Cliniques Universitaires Saint-Luc, Brussels, Belgium, 7Department of Gastroenterology, University of Cambridge, Cambridge, UK, 8Department of Pharmacovigilance and Patient Safety, AbbVie Inc., North Chicago, USA, 9Department of Immunology, AbbVie Inc., North Chicago, USA, 10Department of Data and Statistical Sciences, AbbVie Inc., North Chicago, USA, 11Department of Immunology, AbbVie Deutschland GmbH & Co. KG, Ludwigshafen, Germany, 12Department of Gastroenterology, Amsterdam University Medical Center, Amsterdam, The Netherlands
Background
Efficacy and safety of the IL-23 inhibitor risankizumab (RZB) have been assessed in patients with moderate-to-severe Crohn’s disease (CD) following induction/maintenance treatment.1,2 Responders to RZB in a Phase 2 induction/maintenance study2,3 could enrol in an open-label extension (OLE), NCT02513459.4 Final safety and efficacy results from this RZB OLE study are reported here.
Methods
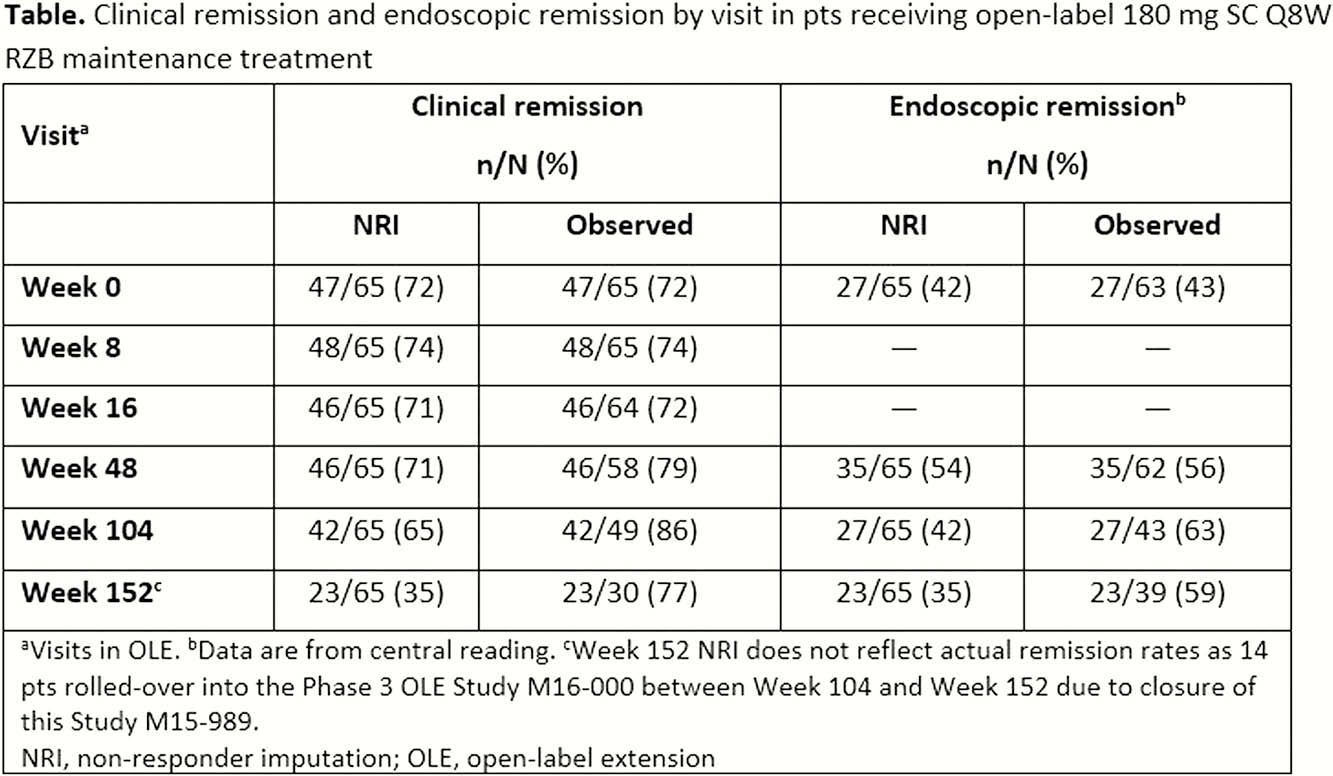
Patients achieving clinical response (CResp) (decrease from baseline [BL] in CD Activity Index [CDAI] ≥100) without remission (CRem) (CDAI <150) after Period 2 (Week 26) or CResp/CRem after Period 3 (Week 52) of the preceding study1 received open-label 180 mg subcutaneous (SC) RZB every 8 weeks (Q8W) for up to 206 weeks. Patients who lost CResp/CRem at screening of the OLE were re-induced with open-label 600 mg IV RZB at Weeks 0, 4, and 8. Patients receiving re-induction treatment only received subsequent 180 mg SC RZB Q8W if they regained CResp/CRem following re-induction. A centrally read ileocolonoscopy was performed yearly. Treatment-emergent adverse events (AEs) were collected up to 20 weeks after the last RZB dose. CRem and endoscopic remission (ER [CD Endoscopic Index of Severity (CDEIS) ≤4 or CDEIS ≤2 for patients with isolated ileitis at BL]) were reported up to Week 152. Non-responder imputation (NRI) and observed case analysis were used for binary endpoints.
Results
Sixty-five patients with CD were enrolled in the OLE, with 4 patients re-induced. At BL of the preceding study, median (range) age was 34 (19–67) years and median (range) disease duration was 10 (2–38) years. Sixty patients (92%) were previously exposed to TNF antagonists. In the OLE, median (range) exposure to RZB was 1014.0 (114–1317) days. Twenty-one (32%) patients prematurely discontinued RZB, including 6 (9%) who had developed an AE. AEs were reported in 60 (92%) patients; 23 (35%) experienced serious AEs. The most common AEs were nasopharyngitis (31%), gastroenteritis (23%), and fatigue (20%). Serious infections were reported in 6 (9%) patients and opportunistic infections in 3 (5%) patients. No tuberculosis, malignancies, or deaths occurred. At Week 0 of the current study, 47 (72%) patients were in CRem and 27 (42%) patients had ER. Both CRem and ER were sustained up to Week 152 (Table).
Conclusion
In this final analysis of patients with CD receiving long-term open-label RZB treatment, the safety profile of RZB remained consistent with previous data² with no new safety signals. Clinical and endoscopic remissions were sustained.
Feagan BG
Feagan BG
Feagan BG
Ferrante M


