OP28 A randomized placebo controlled clinical trial with 5-hydroxytryptophan in patients with quiescent Inflammatory Bowel Disease and fatigue (Trp-IBD)
Truyens, M.(1,2,3);Lobaton, T.(1,3);Peeters, A.(1);Ferrante, M.(4,5);Vermeire, S.(4,5);Bossuyt, P.(6);Pouillon, L.(6);Dewint, P.(7,8);Cremer, A.(9);Peeters, H.(10);Lambrecht, G.(11);Louis, E.(12);Rahier, J.F.(13);Dewit, O.(14);Muls, V.(15);Holvoet, T.(1,16);Vandermeulen, L.(17);Gonzales, G.B.(2,3,18);Laukens, D.(2,3);De Vos, M.(3);
(1)University Hospital Ghent, Department of Gastroenterology, Ghent, Belgium;(2)Ghent University, VIB center for Inflammation Research IRC, Ghent, Belgium;(3)Ghent University, Department of Internal Medicine and Pediatrics, Ghent, Belgium;(4)KU Leuven, Department of Chronic Diseases- Metabolism and Ageing CHROMETA- Translational Research Center for Gastrointestinal Disorders TARGID, Leuven, Belgium;(5)University Hospitals Leuven, Department of Gastroenterology and Hepatology, Leuven, Belgium;(6)Imelda General Hospital, Imelda GI Clinical Research Center, Bonheiden, Belgium;(7)AZ Maria Middelares, Department of Gastroenterology, Ghent, Belgium;(8)UZ Antwerp, Department of Gastroenterology, Antwerp, Belgium;(9)Erasme Hospital, Department of Gastroenterology, Brussels, Belgium;(10)AZ Sint-Lucas, St-Lucas IBD Clinic, Ghent, Belgium;(11)AZ Damiaan, Department of Gastroenterology, Oostende, Belgium;(12)CHU Liège University Hospital, Department of Gastroenterology, Liège, Belgium;(13)CHU UCL Namur, Department of Gastroenterology, Yvoir, Belgium;(14)UCL Saint Luc, Service d’Hépato-Gastroentérologie, Brussels, Belgium;(15)Saint-Pierre University Hospital Center - Université Libre de Bruxelles ULB, Department of Gastroenterology and Endoscopy, Brussels, Belgium;(16)AZ Nikolaas General Hospital, Department of Gastroenterology, Sint-Niklaas, Belgium;(17)Universitair Ziekenhuis Brussel UZ Brussel - Vrije Universiteit Brussel VUB, Department of Gastroenterology-Hepatology, Brussels, Belgium;(18)Wageningen University and Research, Nutrition- Metabolism and Genomics Group - Division of Human Nutrition and Health, Wageningen, Belgium;
Background
Fatigue is highly prevalent in patients with IBD independent of the disease status but treatment options remain limited. A potential mediator in the pathophysiology of fatigue is tryptophan (Trp), a precursor of serotonin. Recently, reduced serum Trp levels have been linked to fatigue in patients with clinically and endoscopically inactive IBD. The aim of the current study was to determine the effect of oral 5-hydroxytryptophan (5-HTP), the direct precursor of serotonin, supplementation on fatigue in patients with inactive IBD.
Methods
This multicentre, randomized, double-blind, cross-over, placebo-controlled trial included fatigued patients with IBD in clinical and biochemical remission (CRP <10mg/L, calprotectin <250 mg/kg), treated with immunosuppressants and/or biologicals. Fatigue was assessed with the fatigue VAS (fVAS, range 0-10) and defined by a fVAS ≥5. Patients were treated in a cross-over manner with 100 mg 5-HTP or placebo bid for two consecutive periods of 8 weeks, without an intermediate washout period. The primary endpoint was the proportion of patients reaching a 20% reduction in fVAS after 8 weeks of intervention (week 8 versus week 0 and week 16 versus week 8). Secondary outcomes were changes in validated FACIT-F score, scores for depression and anxiety and changes in Trp metabolites. The effect of the intervention on the outcomes was evaluated by linear mixed modelling (LMM), with the intervention, period and intervention x period as fixed factors and study participant as random factor.
Results
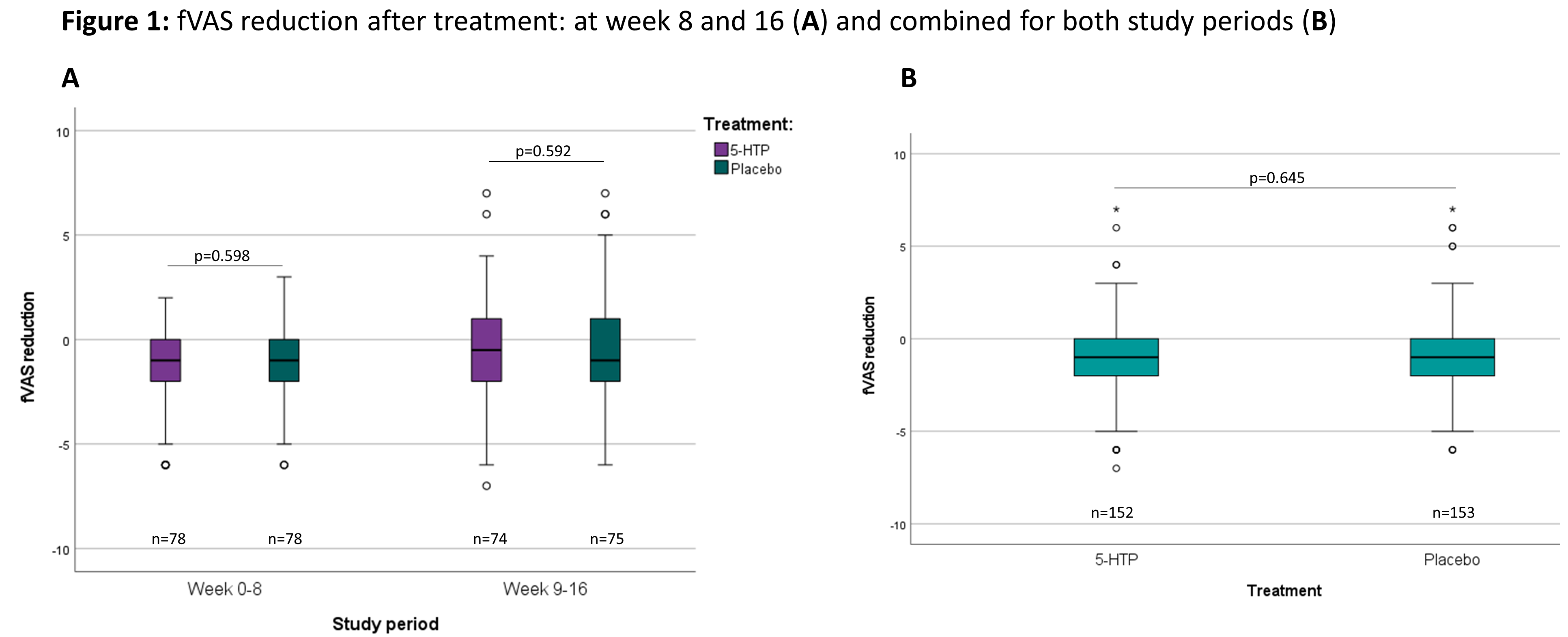
A total of 166 patients were included in 13 Belgian centres between December 2018 and November 2020 (baseline characteristics: Table 1). The dropout rate was 10.8%. The evolution of the fVAS throughout the study was comparable between both study groups and no difference was observed in fVAS reduction between placebo and 5-HTP (Figure 1). The proportion of patients reaching ≥20% reduction in fVAS did not differ between placebo (37.6%) and 5-HTP (35.6%) (p=0.830). The evolution of the other scores for fatigue, depression, anxiety and stress were also similar between placebo and 5-HTP (Table 2). A significant increase in 5-HTP and serotonin serum levels was observed during 5-HTP treatment compared to placebo; whereas serum levels of Trp and kynurenine were comparable. Globally, changes in fVAS were not associated with changes in those metabolites (Figure 2). Adverse events (AEs) were seen in 29.2% and 34.8% of patients under treatment with placebo and 5-HTP respectively (p=0.282).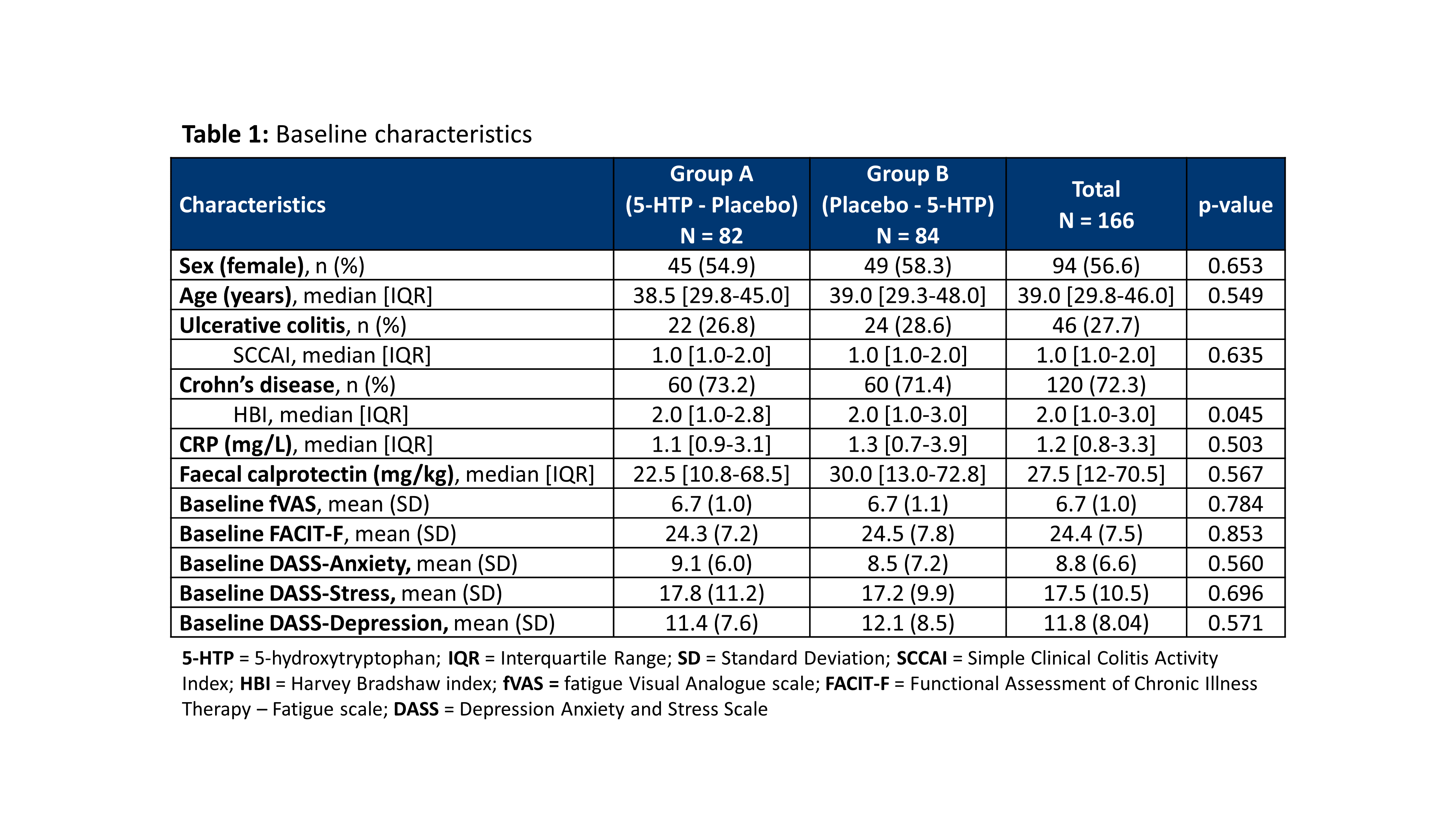


Conclusion
Despite a significant increase in serum 5-HTP and serotonin levels by oral treatment with 5-HTP, 5-HTP did not modulate IBD-related fatigue. Furthermore, treatment with 5-HTP had no impact on depression, anxiety and stress scores.


