OP32 Mincle signalling promotes intestinal mucosal inflammation through induction of macrophage pyroptosis and neutrophil chemotaxis in Crohn’s disease
W. GONG1, K. Guo2, J. Ren3
1School of Medicine, Southeast University, Nanjing, China, 2Department of General Surgery, The First Affiliated Hospital of University of Science and Technology of China, Hefei, China, 3Department of General Surgery, Jinling Hospital, Nanjing, China
Background
Macrophage-inducible C-type lectin (Mincle) signalling plays a proinflammatory role in different organs such as the brain and liver, but its role in intestinal inflammation remains unknown.
Methods
We studied the characteristics of Mincle signalling expression in CD patients and experimental colitis. The functional role of Mincle signalling in the intestine was addressed in experimental colitis models in vivo by using mice with Mincle knock out (Mincle−/−), neutralising anti-Mincle antibody, Mincle pharmacologic agonist and RNA-seq genome expression analysis. Bone marrow-derived macrophages were collected from mice and used to further verify the effect of Mincle signalling in macrophages.
Results
Mincle signalling was significantly elevated in active human CD and experimental colitis, and macrophages were the principal leukocyte subset that up-regulates Mincle signalling. Mincle deficiency ameliorated the colitis by reducing induced macrophage pyroptosis (Figure 1), whereas activation of Mincle with the pharmacologic agonist worsened the intestinal inflammation (Figure 2). Moreover, the ex vivo studies confirmed that Mincle signalling activation promoted and its absence restricted release of proinflammatory cytokines from pyroptosis of macrophage (Figure 3). Finally, Mincle/Syk signalling could promote the production of chemokines to recruit neutrophils by activating Mitogen-Activated Protein Kinase (MAPK) during inflammation (Figure 4).
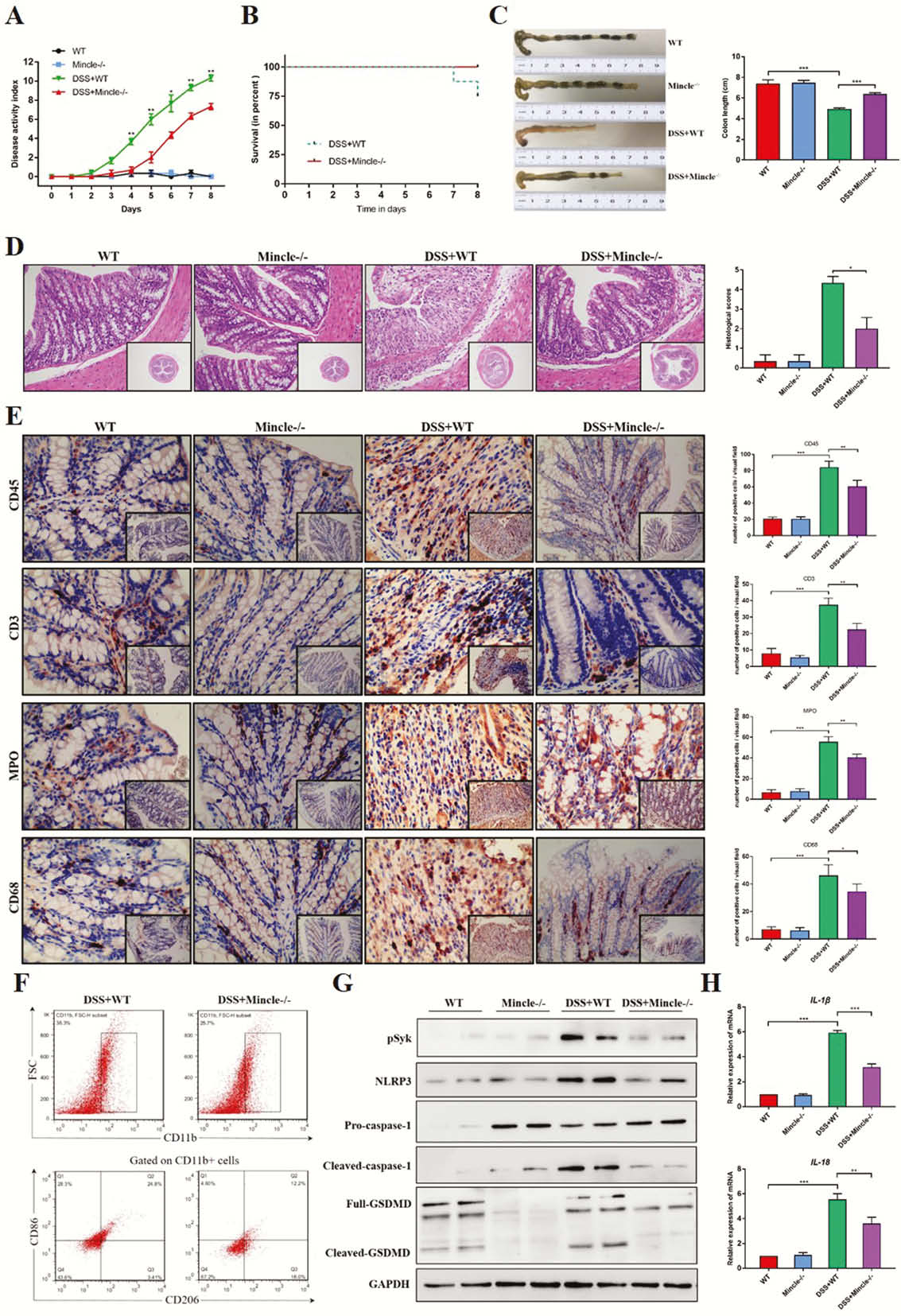
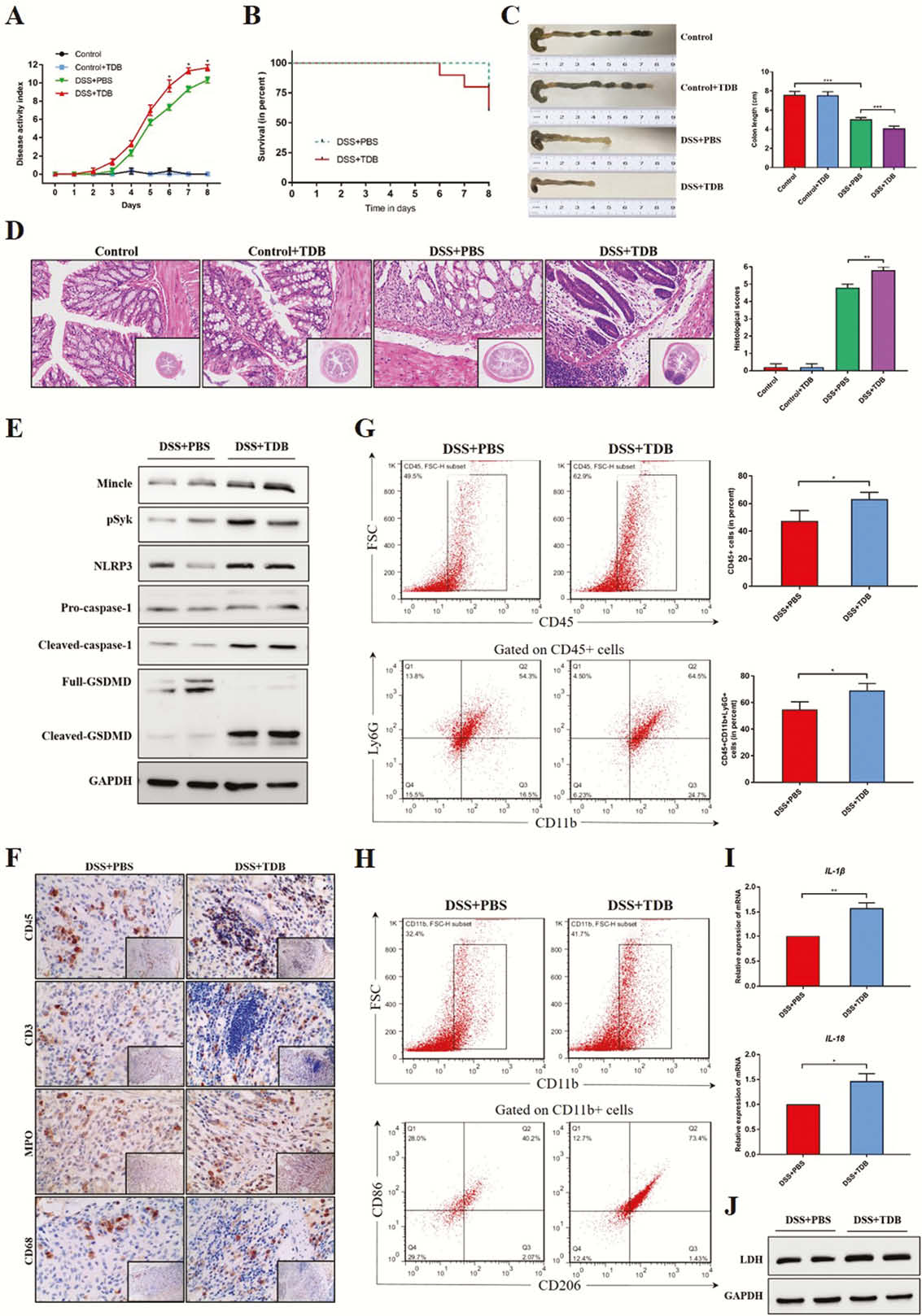
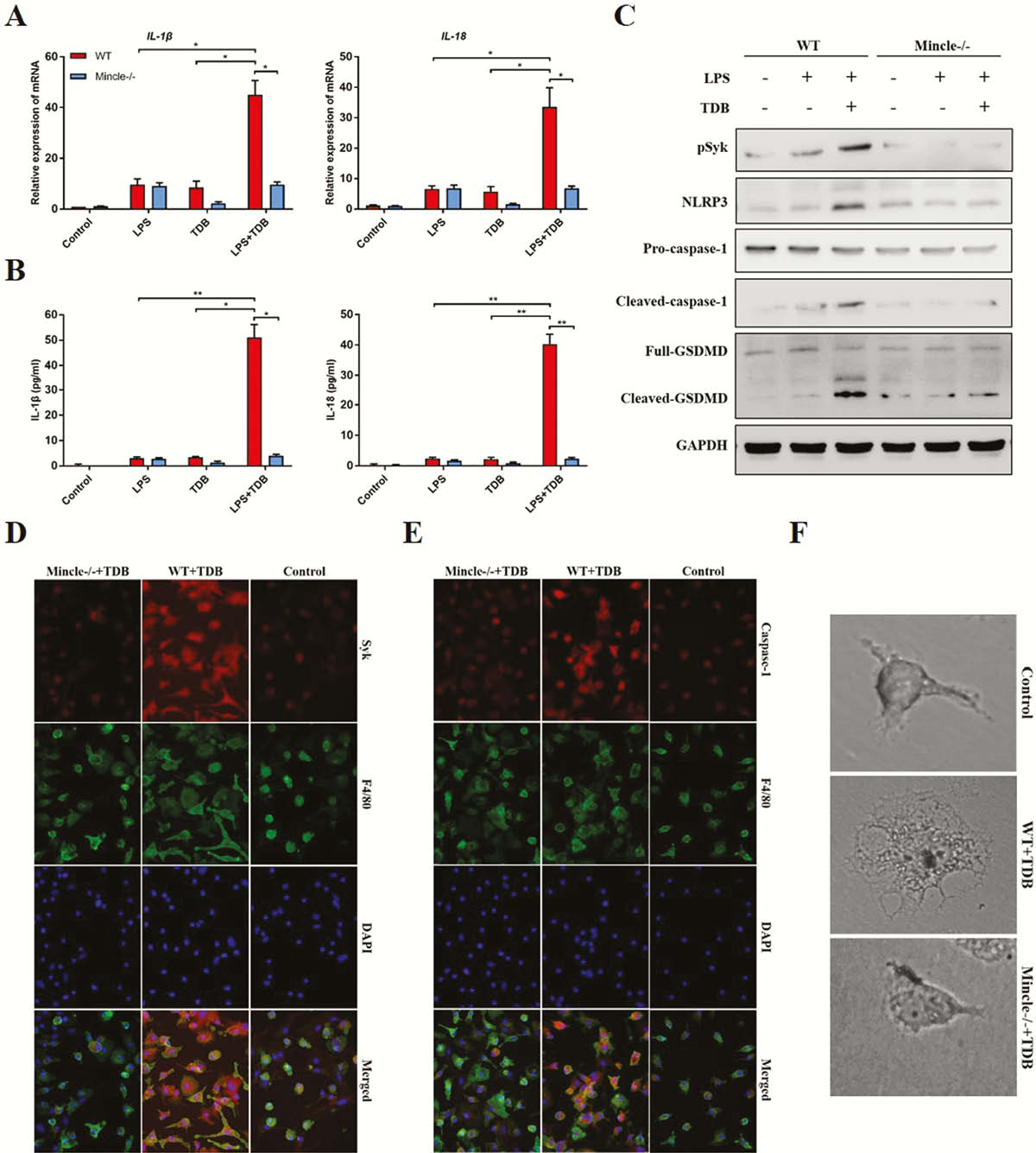
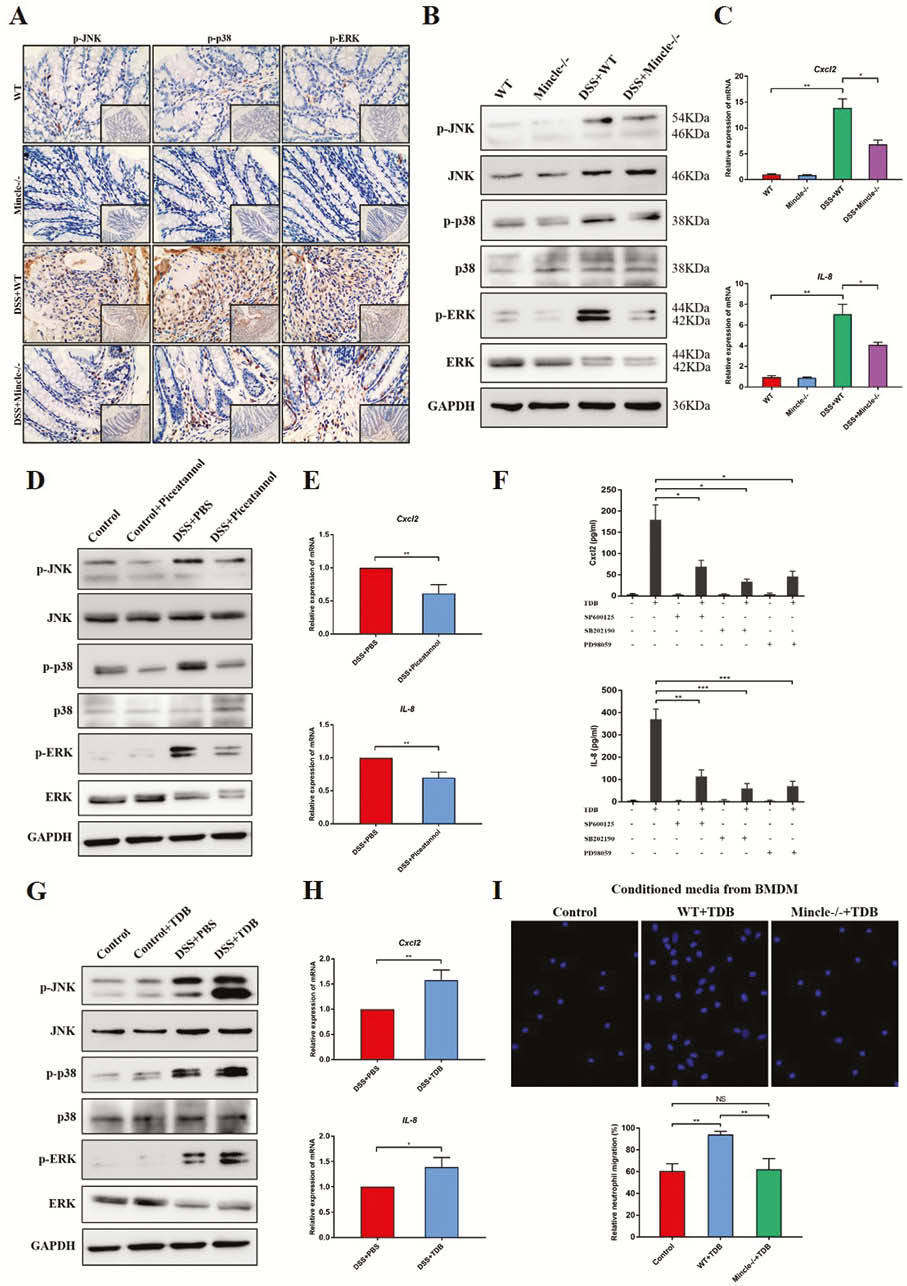
Conclusion
Mincle signalling promotes intestinal mucosal inflammation through induction of macrophage pyroptosis and neutrophil chemotaxis. Modulation of the Mincle/Syk axis emerges as a potential therapeutic strategy to target inflammation and treat CD.


