OP37 Is the withdrawal of anti-tumour necrosis factor in inflammatory bowel disease patients in remission feasible without increasing the risk of relapse? Results from the randomised clinical trial of GETECCU (EXIT)
Chaparro, M.(1)*;García Donday, M.(1);Riestra, S.(2);Lucendo, A.J.(3);Benítez, J.M.(4);Navarro-Llavat, M.(5);Barrio, J.(6);Morales-Alvarado, V.J.(7);Rivero, M.(8);Busquets, D.(9);Leo Carnerero, E.(10);Merino Ochoa, O.(11);Nantes Castillejo, O.(12);Navarro, P.(13);Van Domselaar, M.(14);Gutiérrez Casbas, A.(15);Alonso-Abreu, I.(16);Mejuto, R.(17);Fernández Salazar, L.(18);Iborra, M.(19); Martín-Arranz, M.D.(20); Pineda, J.R.(21); Sampedro, M.J.(22);Serra Nilsson, K.(23);Bouhmidi Assakali, A.(24);Batista, L.(25);Muñoz Villafranca, C.(26);Rodríguez-Lago, I.(27); Ceballos Santos, D.S.(28);Guerra, I.(29);Mañosa, M.(30);Marín Jimenez, I.(31); Vera Mendoza, I.(32);Barreiro-de Acosta, M.(17);Domènech, E.(30);Esteve, M.(25);García-Sánchez, V.(4);Nos, P.(19);Panés, J.(33);Gisbert, J.P.(1);
(1)Hospital Universitario de La Princesa- IIS-Princesa- Universidad Autónoma de Madrid UAM- and CIBEREHD, Gastroenterology Unit, Madrid, Spain;(2)Hospital Universitario Central de Asturias and Instituto de Investigación Sanitaria del Principado de Asturias ISPA, Gastroenterology Unit, Oviedo, Spain;(3)Hospital General de Tomelloso- CIBERehd and Instituto de Investigación Sanitaria de Castilla-La Mancha IDISCAM, Gastroenterology Unit, Tomelloso, Spain;(4)Hospital universitario Reina Sofía and IMIBIC, Gastroenterology Unit, Córdoba, Spain;(5)Hospital de Sant Joan Despí Moisès Broggi, Gastroenterology Unit, Barcelona, Spain;(6)Hospital Universitario Río Hortega- Gerencia Regional de Salud de Castilla y León SACYL, Gastroenterology Unit, Valladolid, Spain;(7)Hospital General de Granollers, Gastroenterology Unit, Granollers, Spain;(8)Hospital Universitario Marqués de Valdecilla and IDIVAL, Gastroenterology Unit, Santander, Spain;(9)Hospital Universitari Dr. Josep Trueta, Gastroenterology Unit, Girona, Spain;(10)Hospital Universitario Virgen del Rocío, Gastroenterology Unit, Sevilla, Spain;(11)Hospital Universitario de Cruces, Gastroenterology Unit, Baracaldo, Spain;(12)Hospital Universitario de Navarra, Gastroenterology Unit, Pamplona, Spain;(13)Hospital Clínico Universitario de Valencia, Gastroenterology Unit, Valencia, Spain;(14)Hospital Universitario de Torrejón and Universidad Francisco de Vitoria, Gastroenterology Unit, Madrid, Spain;(15)Hospital General Universitario Dr Balmis- ISABIAL and CIBERehd, Gastroenterology Unit, Alicante, Spain;(16)Hospital Universitario de Canarias, Gastroenterology Unit, Santa Cruz de Tenerife, Spain;(17)Complexo Hospitalario Universitario de Santiago de Compostela, Gastroenterology Unit, Santiago de Compostela, Spain;(18)Hospital Clínico Universitario de Valladolid and Universidad de Valladolid, Gastroenterology Unit, Valladolid, Spain;(19)Hospital Universitari y Politecnic La Fe, Gastroenterology Unit, Valencia, Spain;(20)Hospital Universitario La Paz- Facultad de Medicina- Universidad Autónoma de Madrid and Instituto de Investigación Hospital universitario La Paz IdiPAZ, Gastroenterology Unit, Madrid, Spain;(21)Xerencia Xestión Integrada de Vigo- SERGAS- Research Group In Digestive Diseases- Galicia Sur Health Research Institute- SERGAS-UVIGO, Gastroenterology Unit, Vigo, Spain;(22)Hospital de Mataró, Gastroenterology Unit, Barcelona, Spain;(23)Hospital Universitari de Bellvitge, Gastroenterology Unit, L'Hospitalet de Llobregat, Spain;(24)Hospital de Santa Bárbara, Gastroenterology Unit, Puertollano, Spain;(25)Hospital Universitari Mutua Terrassa and CIBEREHD, Gastroenterology Unit, Barcelona, Spain;(26)Hospital Universitario de Basurto, Gastroenterology Unit, Bilbao, Spain;(27)Hospital Universitario de Galdakao and Instituto de Investigación Sanitaria Biocruces de Bizkaia, Gastroenterology Unit, Galdakao, Spain;(28)Hospital Universitario de Gran Canaria Dr. Negrín, Gastroenterology Unit, Las Palmas de Gran Canaria, Spain;(29)Hospital Universitario de Fuenlabrada and Instituto de Investigación Hospital Universitario La Paz IdiPAZ, Gastroenterology Unit, Madrid, Spain;(30)Hospital Universitari Germans Trias i Pujol and CIBEREHD, Gastroenterology Unit, Badalona, Spain;(31)Hospital Gregorio Marañón- Instituto de Investigación Sanitaria Gregorio Marañón IiSGM- Facultad de Medicina- Universidad Complutense, Gastroenterology Unit, Madrid, Spain;(32)Hospital Universitario Puerta de Hierro, Gastroenterology Unit, Majadahonda, Spain;(33)Hospital Clinic y Provincial, Gastroenterology Unit, Barcelona, Spain; on behalf of the EXIT Study group of GETECCU
Background
Background: The feasibility of anti-TNF discontinuation in inflammatory bowel disease (IBD) must be proven in clinical trials including patients in clinical, endoscopic, and radiologic remission at the time of anti-TNF withdrawal to make recommendations for clinical practice.
Aims: Primary: to compare the rates of clinical remission at 1 year in patients who discontinue anti-TNF treatment vs. those who continue treatment. Secondary objectives: to know the effect of anti-TNF withdrawal on relapse-free time, mucosal healing and safety; and to identify predictive factors for relapse.
Methods
Prospective, quadruple-blind, multicentre, randomised, controlled trial. Patients with ulcerative colitis (UC) or Crohn’s disease (CD) in clinical remission for > 6 months were randomised to maintain anti-TNF treatment [maintenance arm (MA)] or to withdraw it [withdrawal arm (WA)]. Patients who were on infliximab (IFX) received IFX 5 mg/kg or an intravenous placebo every 8 weeks, while patients on adalimumab (ADA) received subcutaneous ADA 40 mg or placebo every other week. Patients were followed-up until month 12 or up to the time of clinical relapse, whichever came first. Inclusion and exclusion criteria, trial scheme and definitions are summarized in figures 1a, 1b and 1c. Results were analysed by intention-to-treat (ITT) and by per-protocol (PP). Local investigators were blinded to faecal calprotectin (FC) and IFX and ADA trough levels. On-site monitoring was performed to assess data quality.
Results
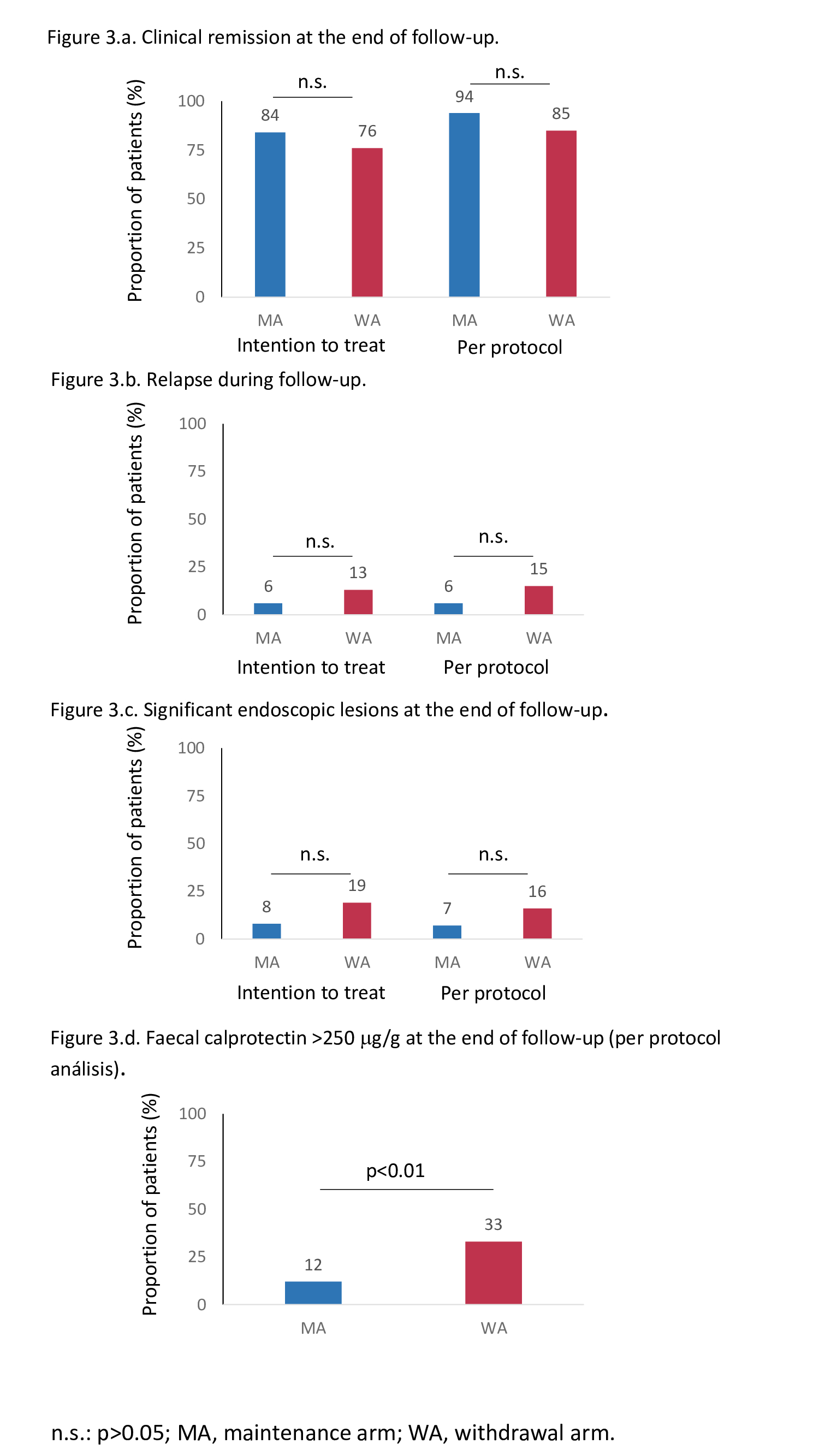
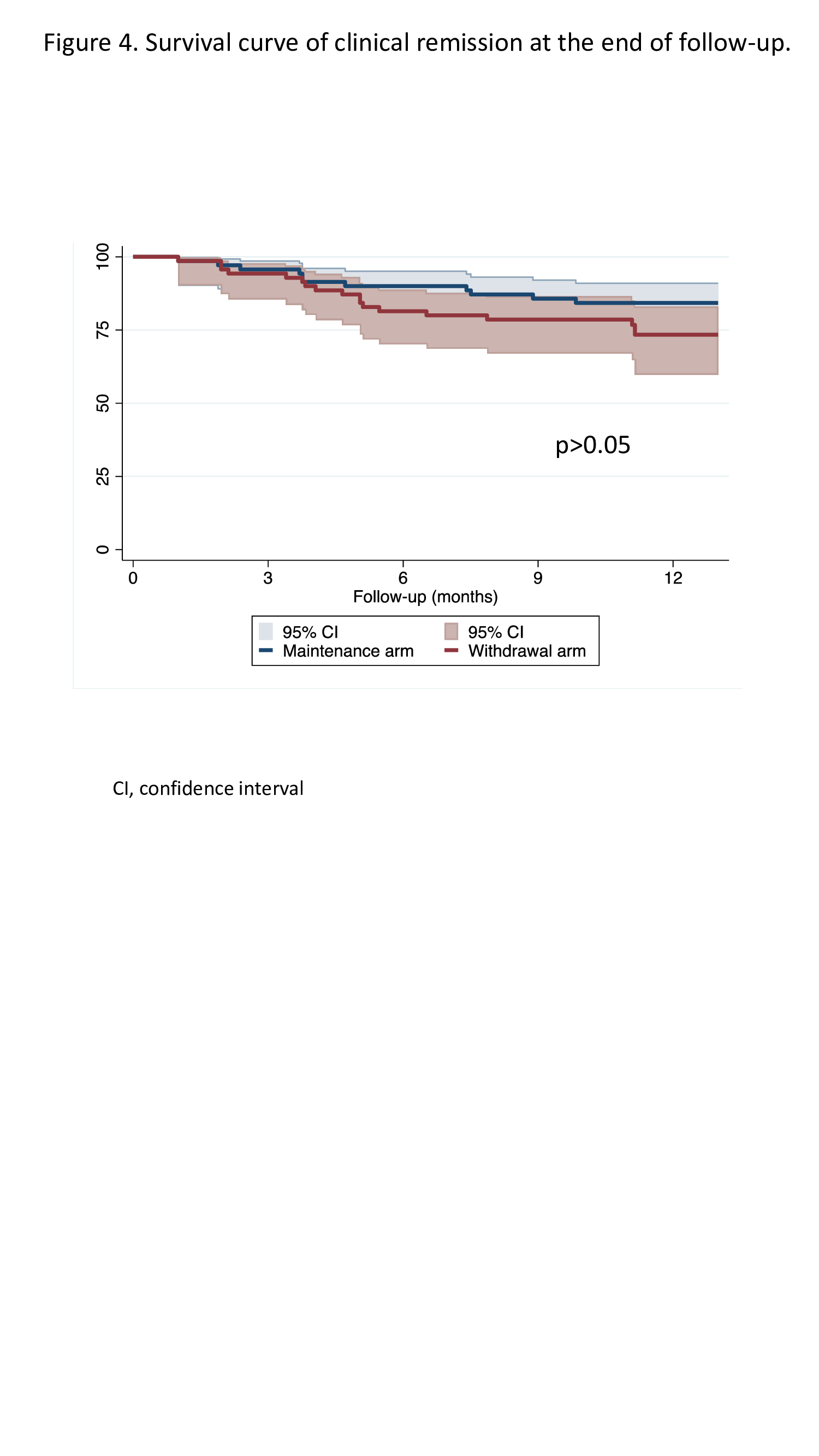
159 patients were screened, from whom 140 were randomised and comprised the ITT cohort: 70 allocated to the MA and 70 to the WA. Fifteen patients dropped out before the end of follow-up (12 months or relapse), leaving 63 patients in the MA and 62 patients in the WA for the PP analysis. The characteristics of patients in the MA and WA were similar (figure 2). The proportions of patients who maintained clinical remission -59/70 (84%), 95% confidence interval (CI)=74-92% in the MA vs. 53/70 (76%), 95%CI=64-85% in the WA- and who remained without significant endoscopic lesions at the end of follow-up were similar between groups (figures 3a, 3b, 3c). Only the proportion of patients with FC >250 mg/g was higher in the WA at the end of follow-up (figure 3d). Maintenance of clinical remission was no different between groups (figure 4). The same percentage of patients in both groups had at least one adverse event (69%). The proportion of patients with serious adverse events was also similar between groups (4% in MA vs. 7% in WA).
Conclusion
Anti-TNF withdrawal in selected IBD patients in clinical, endoscopic, and radiologic remission could be feasible without an increase in the risk of clinical relapse. Long-term follow-up of these patients is warranted.


