OP39 Treatment of ulcerative colitis With AMT-101, a novel oral interleukin-10 immunomodulatory fusion biologic that traffics across the intestinal epithelium
R. Mrsny1, B. Kanwar2, T. Mahmood3
1Applied Molecular Transport, Platform and Biology, South San Francisco, USA, 2Applied Molecular Transport, Clinical Development, South San Francisco, USA, 3Applied Molecular Transport, CEO, South San Francisco, USA
Background
While different chronic inflammatory diseases can be correlated with distinct pro-inflammatory cytokines, interleukin-10 (IL-10) represents a central anti-inflammatory cytokine capable of modulating many pro-inflammatory signals. Previous clinical efforts to capture the benefit of IL-10 in suppressing the pro-inflammatory state in inflammatory bowel disease (IBD) patients have been limited by dose-limiting systemic side effects.
Methods
We have designed a chimaera of human IL-10 genetically fused to a non-toxic and poorly immunogenic fragment of the cholix exotoxin, termed AMT-101, that demonstrated rapid receptor-mediated transcytosis
Results
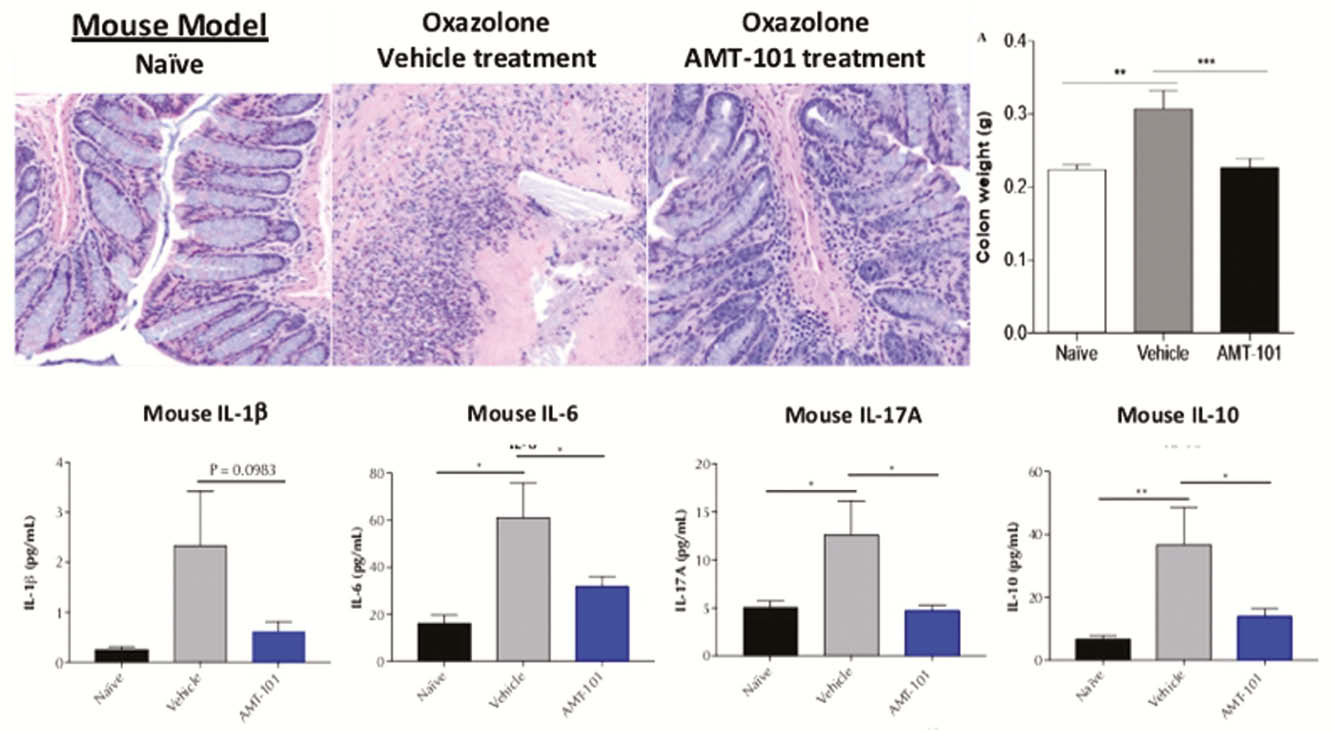
Histological changes of colonic tissue associated with oxazolone-induced colitis was blocked by the oral gavage of AMT-101. Increases in serum levels of pro-inflammatory cytokines IL-1b, IL-6, and IL-17A were blunted by AMT-101 treatment. Remarkably, endogenous IL-10 increased in this model in an attempt to correct inflammation, but this was also decreased by the delivery of AMT-101. Cynomolgus monkeys dosed orally with AMT-101 capsules showed very low serum levels compared with those observed after IV injection of 0.5 mg/kg AMT-101. Strikingly, serum levels of IL-1 receptor antagonist (IL-1RA) as an anti-inflammatory PD marker were increased to a greater extent following oral capsule dosing compared with IV administration.
Conclusion
These studies provide strong pre-clinical evidence that AMT-101 can effectively reach the intestinal


