OP40 Analysis of clinical features associated with favourable outcomes from ustekinumab treat-to-target strategy in Crohn’s Disease patients in the STARDUST trial
Danese, S.(1);Vermeire, S.(2);Dignass, A.(3);Panés, J.(4);D'Haens, G.(5);Magro, F.(6,7);Le Bars, M.(8);Nazar, M.(9);Lahaye, M.(10);Ni, L.(11);Bravatà, I.(12);Gaya, D.R.(13);Peyrin-Biroulet, L.(14)
(1)Humanitas University, IBD Center, Milan, Italy;(2)University Hospitals Leuven, Department of Gastroenterology, Leuven, Belgium;(3)Agaplesion Markus Hospital, Department of Medicine I, Frankfurt/Main, Germany;(4)Hospital Clinic of Barcelona- IDIBAPS- CIBERehd, Department of Gastroenterology, Barcelona, Spain;(5)University of Amsterdam, Amsterdam Medical Center, Amsterdam, The Netherlands;(6)Institute for Molecular and Cell Biology- Faculty of Medicine University of Porto, Department of Pharmacology & Therapeutics, Porto, Portugal;(7)Hospital de São João, Department of Gastroenterology, Porto, Portugal;(8)Janssen-Cilag, Medical Affairs, Issy-les-Moulineaux, France;(9)Janssen-Cilag Polska Sp. z .o.o., Medical Affairs, Warsaw, Poland;(10)Janssen-Cilag BV, Medical Affairs, Breda, The Netherlands;(11)Janssen Cilag Russia, Medical Affairs, Moscow, Russian Federation;(12)Janssen-Cilag, Medical Affairs, Milan, Italy;(13)Glasgow Royal Infirmary, Department of Gastroenterology, Glasgow, United Kingdom;(14)University Hospital of Nancy- University of Lorraine, INSERM Unité 954 and Department of Hepato-Gastroenterology, Houdemont, France
Background
The 48-week (W) interventional STARDUST trial assessed whether a treat-to-target (T2T) strategy using ustekinumab (UST) may optimize Crohn’s disease (CD) outcomes; primary efficacy and safety data have been reported before.1 Here we assessed which patient (pt) subgroups may benefit from T2T vs standard of care (SoC) in achieving endoscopic response after 1 year of UST treatment.
Methods
Adult pts with moderate–severely active CD (CD activity index [CDAI] 220–450) and Simple Endoscopic Score in CD [SES-CD] ≥3) who failed conventional therapy and/or 1 biologic were included. Pts received iv, weight-based UST ~6 mg/kg at W0 (baseline [BL]); then SC UST 90 mg at W8. At W16, CDAI 70 responders were randomized (1:1) to T2T or SoC arms. Pts in the T2T arm were assigned to SC UST q12w or q8w based on 25% improvement in SES-CD score vs BL. From W16–48, UST dose was further intensified up to q4w if the following were not met: CDAI <220 and ≥70-point improvement from BL, and C-reactive protein ≤10 mg/L or faecal calprotectin (FCal) ≤250 µg/g. Pts who failed treatment target despite UST q4w were discontinued. In the SoC arm, UST dose was assigned based on EU SmPC (q12w or q8w). We report the treatment effect for the primary endpoint (endoscopic response [≥50% improvement in SES-CD score vs BL] at W48), evaluated for subgroups of pts, based on demographics at BL. For each subgroup, the odds ratio (OR) and 95% confidence interval (CI) of T2T vs SoC were provided based on the logistic regression model that included treatment arm and stratification factors (prior exposure to biologics [none or 1] and SES-CD score [≤16, >16] at BL) as independent variables.
Results
Of 500 pts enrolled, 441 were randomized to T2T (n=220) or SoC (n=221); 79.1% and 87.3% completed W48. At W48, pts randomized to T2T were more likely to achieve endoscopic response compared to SoC (p<0.05), if they had at BL: (i) longer disease duration (>median [79.1 months]; OR 2.2; 95%CI 1.17–3.94); (ii) clinically moderate disease (CDAI ≤300; OR 1.7; 95%CI 1.03–2.76); (iii) normal FCal (≤250; OR 3.0; 95%CI 1.22–7.56), (iv) endoscopically active CD (SES-CD ≥4 for ileal or ≥6 for colonic and/or ileocolonic disease; OR 1.8; 95%CI 1.10–2.91); and (v) history or presence of strictures/fistula or occurrence of an intra-abdominal abscess (OR 2.3; 95%CI 1.06–5.01 and OR 3.5; 95%CI 1.07-11.19, respectively; Figure 1).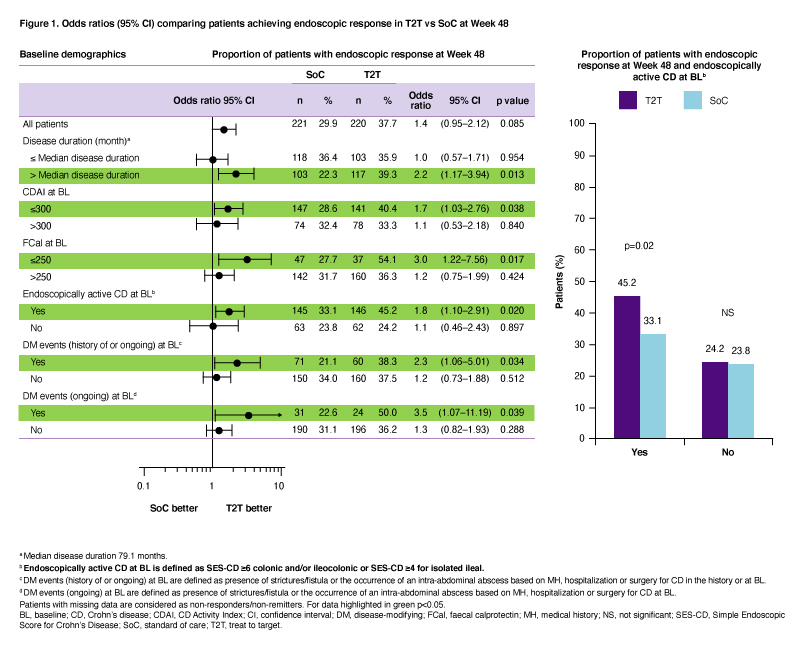
Conclusion
T2T was more effective than SoC (p<0.05) in achieving endoscopic response after 1 year of UST treatment in certain subgroups including pts with higher endoscopic scores at BL and those with history/presence of bowel damage.
1. Danese S, et al. United European Gastroenterol J. 2020;8:1264–1265 (Abstract LB11).


