P035 NUDT15 variance increases DNA-incorporated thiopurine metabolites and lymphocyte apoptosis in patients with inflammatory bowel disease
H. Kiyohara1, T. Toyonaga1, S. Kuronuma2, A. Ueno3, S. Okabayashi3, R. Ozaki1, O. Takeuchi2, S.A. Coulthard4, C.P.F. Redfern4, H. Terai5, Y. Tanaka6, M. Nakano1, T. Hibi3, T. Kobayashi3
1Department of Gastroenterology/Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Tokyo, Japan, 2Department of Research, Kitasato University Kitasato Institute Hospital, Tokyo, Japan, 3Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Tokyo, Japan, 4The Newcastle Cancer Centre, Northern Institute for Cancer Research, Newcastle University, Newcastle upon Tyne, UK, 5Department of Respiratory Medicine, Kitasato University Kitasato Institute Hospital, Tokyo, Japan, 6Department of Clinical Pharmacy, Center for Clinical Pharmacy and Sciences, Kitasato University School of Pharmacy, Tokyo, Japan
Background
A novel thiopurine metabolizing enzyme, nucleotide diphosphate-linked moiety X-type motif 15 (NUDT15) was associated with drug-induced leukopenia in patients with non-synonymous genetic polymorphisms. Thiopurine-induced leukopenia in Japanese patients with genetic variance in NUDT15 (c.415C>T) appears to be independent of the 6-thioguanine nucleotide concentration in red blood cells. However, detailed molecular mechanism how NUDT15 variance causes thiopurine-induced leukopenia remains unclear and NUDT15-associated subcellular thiopurine metabolism has not been investigated in patients with inflammatory bowel diseases (IBD).
Methods
DNA-incorporated deoxythioguanosine (dTG) was measured in the peripheral blood mononuclear cells (PBMCs) of Japanese patients with IBD under thiopurine treatment. Association of a single-nucleotide polymorphism for NUDT15 (c.415C>T) with dTG in PBMCs (dTGPBMC) was examined. Peripheral blood T lymphocytes were cultured
Results
NUDT15 variants had significantly higher dTGPBMC per thiopurine dosage than non-variants (homozygous variants (TT) vs. heterozygous variants (CT) vs. non-variants (CC), 4418.0 vs. 663.0 vs. 295.3 dTG mol/106 moles dA per mg/kg/day of 6-MP (Figure A)). dTGPBMC and peripheral lymphocyte counts showed a negative correlation (
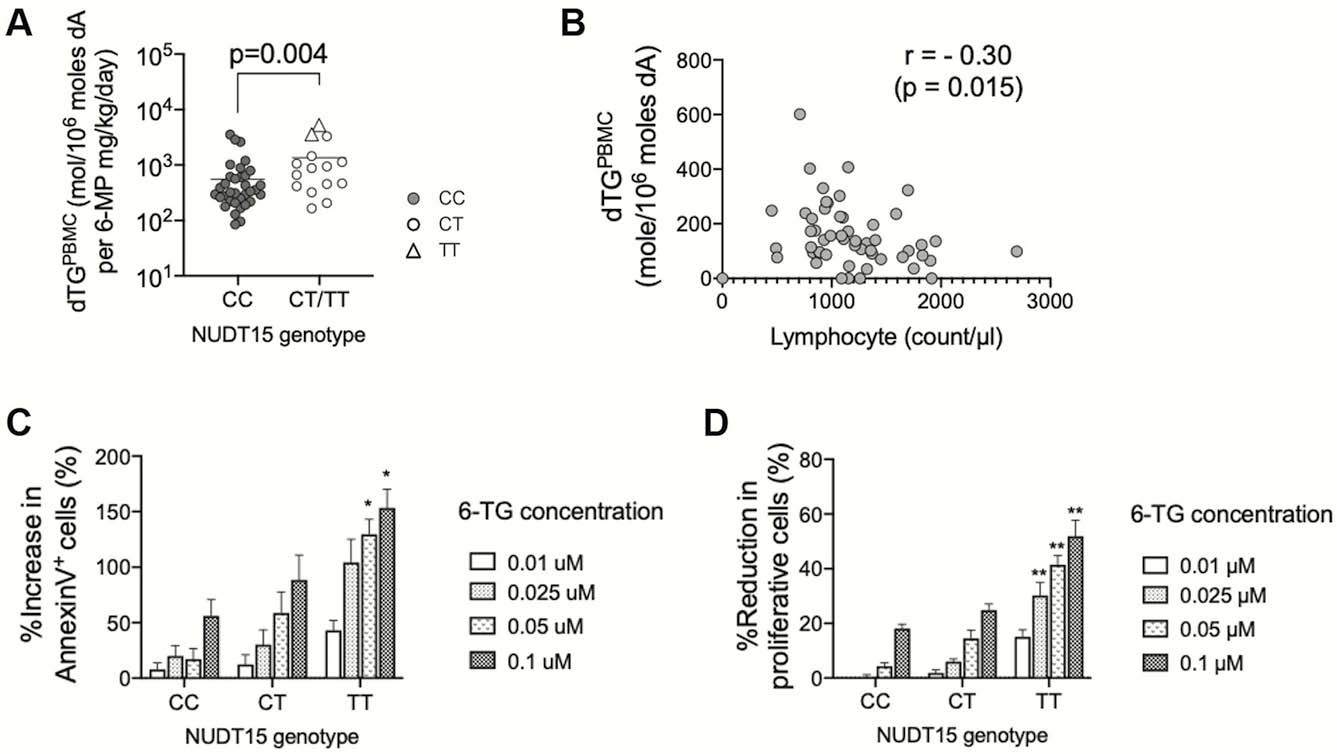
Conclusion
DNA-incorporated dTG affected by NUDT15 genotypes induces T lymphocyte apoptosis in patients with IBD.
- Posted in: Poster presentations: Basic Science 2020


