P039 The disappearance of cell death brakes in inflammatory bowel disease: a step towards molecular healing
Al-Ani, A.(1)*;Samson, A.(2);Rickard, J.(3);Christensen, B.(4);Murphy, J.(2);
(1)The Royal Melbourne Hospital Melbourne Health, Gastroenterology, Melbourne, Australia;(2)Walter and Eliza Hall Institute of Medical Research, Inflammation Division, Melbourne, Australia;(3)Royal Melbourne Hospital, Anatomical Pathology, Melbourne, Australia;(4)Royal Melbourne Hospital, Gastroenterology, Melbourne, Australia;
Background
The core mechanistic driver underpinning the relapsing and progressive nature of inflammatory bowel disease (IBD) remains unknown with current therapies targeting immune or inflammatory pathways. Our in-depth analysis of regulated death pathways remarkably reveals that cell death brakes are removed in intestinal IBD tissue, indicating cells remain “primed” to die regardless of endoscopic and histologic healing. Characterisation of cell death is essential to confirm our hypothesis that disinhibition of programmed cell death is a rudimentary molecular defect in IBD. Restoration of cell death brakes may be intrinsic to molecular healing and novel drug development.
Methods
Intestinal biopsies are collected from inflamed, marginal and non-inflamed regions during endoscopy for patients with IBD and matched with those without IBD (“controls”). Tissue is processed to quantify cell death signals and changes to the local microbiome using immunoblotting, immunohistochemistry, immunofluorescence, organoid preparation and RNA sequencing (Fig. 1). Laboratory data are correlated with clinical, biochemical, endoscopic and histologic parameters.
Results
To date, 59 patients have been recruited: 29 controls; 31 IBD (13 Crohn’s disease, 16 ulcerative colitis, 2 pouchitis). Interim analyses of 20 patients show loss of cell death brakes (cIAP1, cIAP2, cFLIP) suggesting disinhibition of cell death even in endoscopically and histologically normal IBD tissue (Fig. 2). Concurrently, there is increased apoptosis (characterised by cleaved caspase-3) in IBD intestinal tissue regardless of inflammatory activity (Fig. 3). Necroptosis, a programmed lytic form of cell death, is active in steady state conditions in controls. Additionally, necroptosis appears markedly elevated in stricturing and penetrating Crohn’s disease compared to other subsets of IBD (Fig. 2).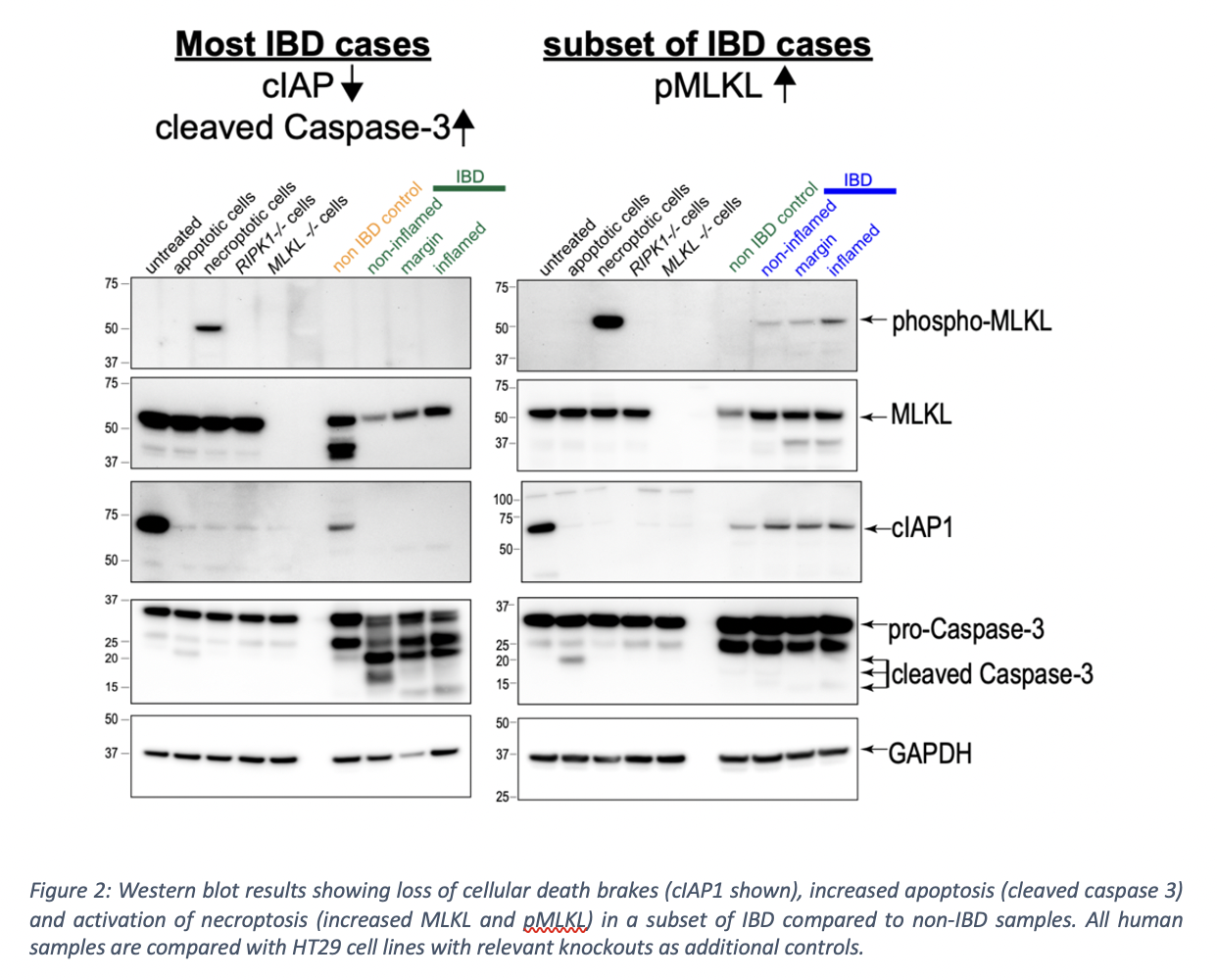

Conclusion
This study demonstrates for the first time that cellular death brakes are removed in intestinal tissue in IBD, despite deep remission with seemingly effective biologic therapy. Disinhibition of programmed cell death is likely intrinsic to the inflammatory sequence underlying disease recurrence and progression. Restoration of cell death brakes may hence be key to achieving long-term, relapse-free remission and should be considered an aspirational goal for both drug development and achievement of molecular healing. Our results challenge current dogma based on murine data that necroptosis occurs in the absence of apoptosis and that necroptosis is universally increased in all forms of IBD. Instead, we propose that a balance of apoptosis and necroptosis is essential in intestinal homeostasis with endotyping of disease based on cell death profile a feasible goal towards precision medicine in IBD.


