P054 LHFPL3-AS2 long non-coding (lncRNA) is reduced in mucosal biopsies of Crohn Disease, and its reduction in tissue culture disrupts epithelial polarity
Sosnovski, K.(1);BenShoShan , M.(2);Braun , T.(3);Barshack , I.(4);Denson , L.(5);Haberman , Y.(6);
(1)Tel-Aviv University, Sackler, Tel-Aviv, Israel;(2)Tel-Aviv, Sackler, Tel-Aviv, Israel;(3)Sheba Medical Centre, Gastroenterology, Ramat-Gan, Israel;(4)Sheba Medical Center, Pathology, Ramat-Gan, Israel;(5)Cincinnati Children’s Hospital, Gastroenterology, Cincinnati, United States;(6)Sheba Medical Center, Gastroenterology, Ramat-Gan, Israel;
Background
Disruption of apico-basal polarity attenuate Intestinal epithelia barrier and is part of the Epithelial–mesenchymal transition process linked with Crohn Disease (CD). We have defined widespread dysregulation of long non-coding RNAs (lncRNA), known to have cellular regulatory role, in treatment naïve CD ileum including reduction of LHFPL3-AS2.
Aim: We aimed to explore the effect of LHFPL3-AS2 reduced expression in the context of CD pathogenesis.
Methods
dCas9/CRISPRi was used for LHFPL3-AS2 knock-down in Caco-2 cells. Transcriptomics, qPCR, western blots, and confocal microscopy was used to evaluate the effect of LHFPL3-AS2 knock-down.
Results
LHFPL3-AS2 expression in isolated ileum and ascending colon epithelia from controls was significantly higher than in the sigmoid colon, with LHFPL3-AS2 reduction in the ileum of CD cases vs. controls (p<0.01). LHFPL3-AS2 co-expression analyses showed enrichment for genes linked with cytoskeleton and tight junction. Reducing LHFPL3-AS2 expression by 2 gRNAs, strikingly resulted in abnormal cell morphology. Unlike control cells that developed polarized cysts in 3D, the LHFPL3-AS2 knockdown cells failed to form the luminal cavity within the cyst, which was coupled with abnormal distribution of F-actin throughout the cell membrane, rather than primarily in the luminal area (Fig. 1). mRNA-seq analysis on LHFPL3-AS2 knockdown and controls detected 584 differentially expressed genes (fold change>=1.5 and FDR corrected p<0.05). Similarly, the down regulated genes showed enrichment for pathways (p<E-4) linked with apical part of cell membrane, actin filament bundle, microvillus, regulation of morphogenesis of an epithelium, adherent junctions, and b-catenine-TCF4 complex. Staining for CTNNB1, JAM-A, e-Cadherin (CDH1), and CDH11 showed abnormal organization and formation of no lumen or multiple smaller lumens within the cysts, overall suggesting abnormal epithelial polarity organization. Using String network analyses tool on the down-regulated genes, we identified CTNNB1, a key downstream component of the canonical Wnt signaling pathway, as a central node in the network suggesting potential attenuation of downstream Wnt signaling (Fig. 2). Activating the Wnt/β-catenin pathway, via LiCl, showed more substantial reduction of CTNNB1, and tight junction proteins - CDH1, CDH11, indicating that the LHFPL3-AS2 knock-down further inhibited the ability of the cells to respond to WNT activation.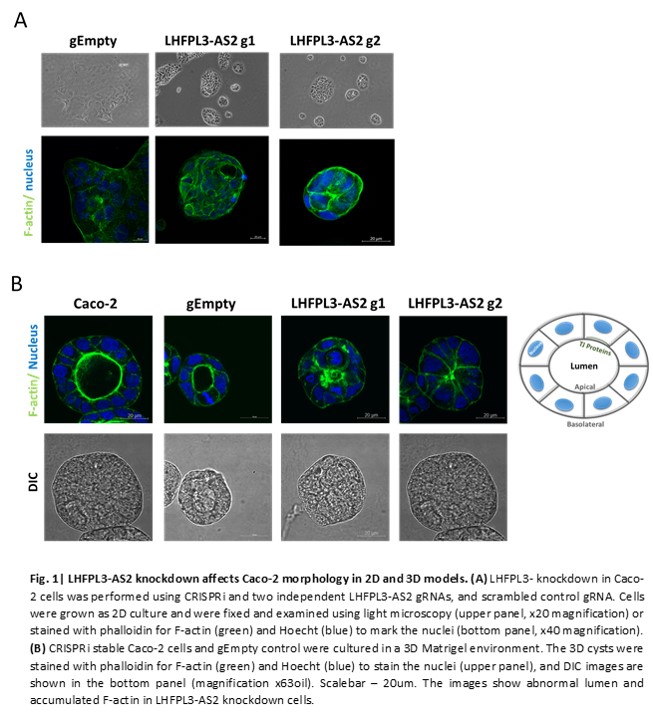

Conclusion
Reduced LHFPL3-AS2 in CD epithelia may contribute to attenuated epithelial polarity. Boosting LHFPL3-AS2 lncRNA expression to enable proper epithelial polarity may reverse the initial step in the CD-associated Epithelial–mesenchymal transition process.


