P062 Dose-dependent differential effects of vedolizumab therapy on adhesion of regulatory and effector T cells
E. Becker, M. Wiendl, A. Schulz-Kuhnt, I. Atreya, R. Atreya, M. Neurath, S. Zundler
University of Erlangen-Nuremberg, Department of Medicine 1, Kussmaul Campus for Medical Research and Translational Research Center, Erlangen, Germany
Background
Vedolizumab has emerged as an important pillar of treatment in inflammatory bowel disease (IBD). However, for unknown reasons, not all patients respond to therapy. Earlier clinical studies suggested decreased response rates in the highest compared with medium dosage groups. Interestingly, vedolizumab has been shown to inhibit the homing of both regulatory (Treg) and effector T (Teff) cells and previous data from our group suggested different effect sizes in both populations. Thus, we hypothesised that the non-linear exposure–efficacy correlation might be explained by dose-dependent differential effects of vedolizumab on Treg and Teff homing. Therefore, we studied functional effects of different vedolizumab exposure levels on Treg and Teff cell trafficking.
Methods
The α4β7 expression on different human T-cell subsets as well as the binding characteristics of vedolizumab to these cells at different exposure levels was analysed via flow cytometry. Functional effects of different vedolizumab concentrations on the adhesion of Tregs and Teffs to mucosal addressin cell adhesion molecule 1 (MAdCAM-1) were analysed using dynamic
Results
We found a preferential binding of vedolizumab to Tregs at an exposure with 0.4 µg/ml vedolizumab that shifted to a preferential binding to Teffs at an exposure with 10 µg/ml. Further increase of vedolizumab to 50 µg/ml led to equal binding to Tregs and Teffs (Figure 1). Consistently, at 10 µg/ml, dynamic adhesion of Tregs to MAdCAM-1 was increased compared with Teffs, but no difference was noted at 50 µg/ml. Additionally, a higher number of Treg compared with Teff cells were able to transmigrate in a MAdCAM-1-dependent manner at a concentration of 10 µg/ml vedolizumab. Preliminary data from homing experiments in a humanised mouse model and from IBD patients treated with vedolizumab support the notion that differential binding preferences depending on the exposure level can also be observed
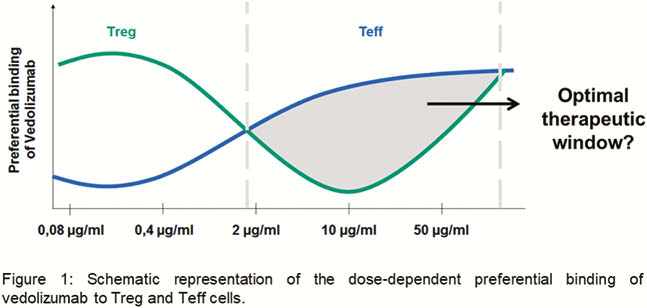
Conclusion
Our findings support a dose-dependent differential binding of vedolizumab to different T-cell subpopulations and suggest that an optimal ‘window’ of exposure exists, in which effects on Teffs predominate over Tregs. While offering a potential explanation for earlier findings in dose-ranging studies, our data might lay the basis for the establishment of individualised dose optimisation in IBD patients.


