P067 Proteins citrullination and Crohn’s disease: PAD4 but not PAD2 is a strong marker of ileal inflammation
G. Dragoni1,2, B. Creyns2,3, G. De Hertogh4, B. Verstockt2,5, W.J. Wollants2, B.J. Ke2, L. Marcellis4, G. Matteoli2, A. D’Hoore6, A. Galli1, M. Ferrante2,5, S. Vermeire2,5
1University of Florence, Clinical Gastroenterology Unit- Department of Biomedical Clinical and Experimental Sciences ‘Mario Serio’, Firenze, Italy, 2KU Leuven, Department of Chronic Diseases, Metabolism and Ageing, Translational Research Center for Gastrointestinal Disorders TARGID, Leuven, Belgium, 3KU Leuven, Department of Microbiology, Immunology and Transplantation, Allergy and Clinical Immunology Research Group, Leuven, Belgium, 4KU Leuven, Department of Imaging and Pathology, Translational Cell and Tissue Research, Leuven, Belgium, 5University Hospitals Leuven, Department of Gastroenterology and Hepatology, Leuven, Belgium, 6University Hospitals Leuven, Department of Abdominal Surgical Oncology, Leuven, Belgium
Background
Citrullination is a post-translational modification of proteins, mediated by enzymes called PAD (peptidylarginine deiminases). The immune system can attack citrullinated proteins, leading to autoimmune diseases such as rheumatoid arthritis, multiple sclerosis and ulcerative colitis, and the activity of PAD2 and PAD4 in innate immune cells has been demonstrated for these disorders. Recently, high levels of PAD2 have been described in activated fibroblasts in the context of liver fibrosis. We therefore investigated the role of PAD2 and PAD4, both in inflammatory and fibrotic contexts of ileal Crohn’s disease (CD).
Methods
We obtained ileal transmural samples from patients operated for stricturing ileal CD. Three different macroscopic areas within each resection specimen (i.e. proximal normal ileum, inflamed ileum and fibrotic ileum) were selected and histologically confirmed by an expert pathologist. Patients undergoing ileocolic resection for other conditions (e.g. right colon cancer) and with healthy terminal ileum were used as controls. For each region (normal CD, inflamed CD, fibrotic CD and control), immunohistochemistry (IHC), RNA and protein evaluations for PAD2 and PAD4 were performed. Multiplex immunofluorescence (IF) for PAD2, PAD4, myeloperoxidase, neutrophil elastase, CD68, vimentin and α-smooth muscle actin were carried out to investigate the enzymes-expressing cells. Additional IF was performed to study citrullinated histone 3 (H3cit) expression, the product of PAD4 activity in neutrophils and component of neutrophil extracellular traps (NETs). Statistical analysis was carried out with Kruskal–Wallis test and
Results
Resection specimens from 13 CD and 11 controls were included. IHC and IF showed an increased expression of both PAD2 and PAD4 in the neutrophils of inflamed areas, in cytoplasm and nucleus, respectively (Figure 1). Activated fibroblasts (vimentin+ and α-smooth muscle actin+) were negative for both enzymes. PAD4 mRNA expression was increased in inflamed tissue (
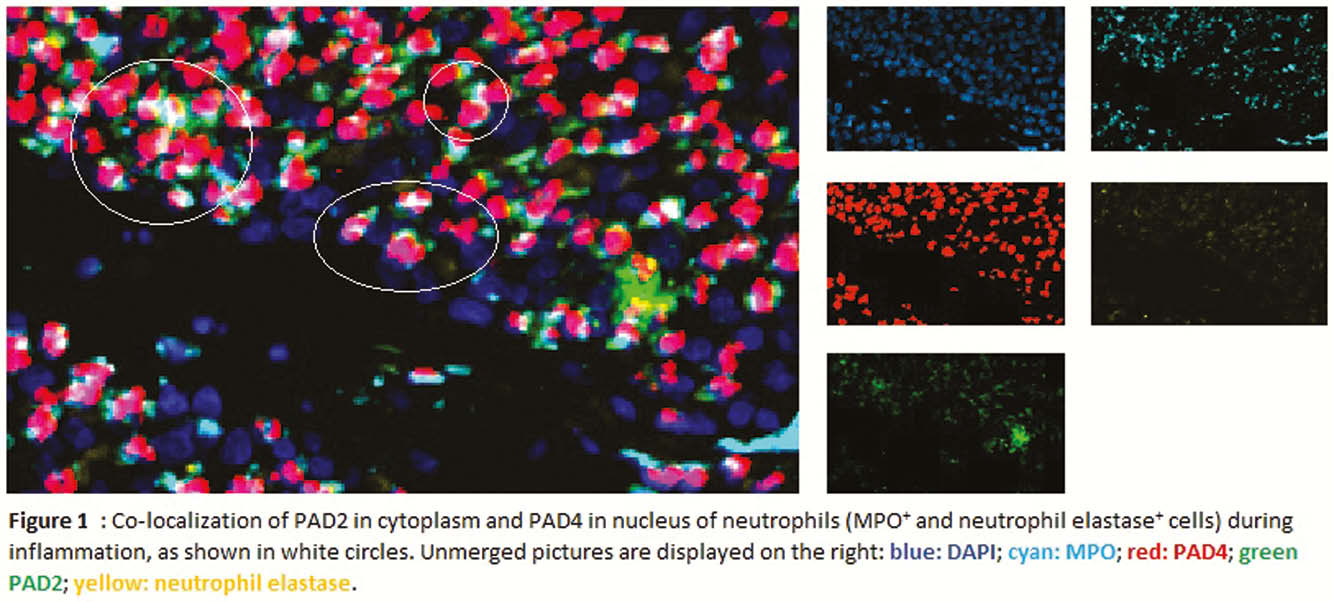
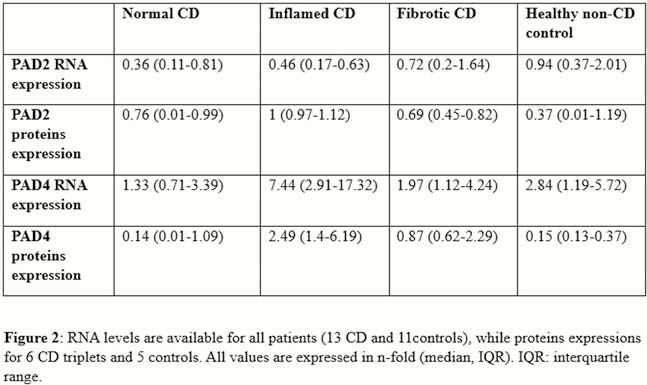
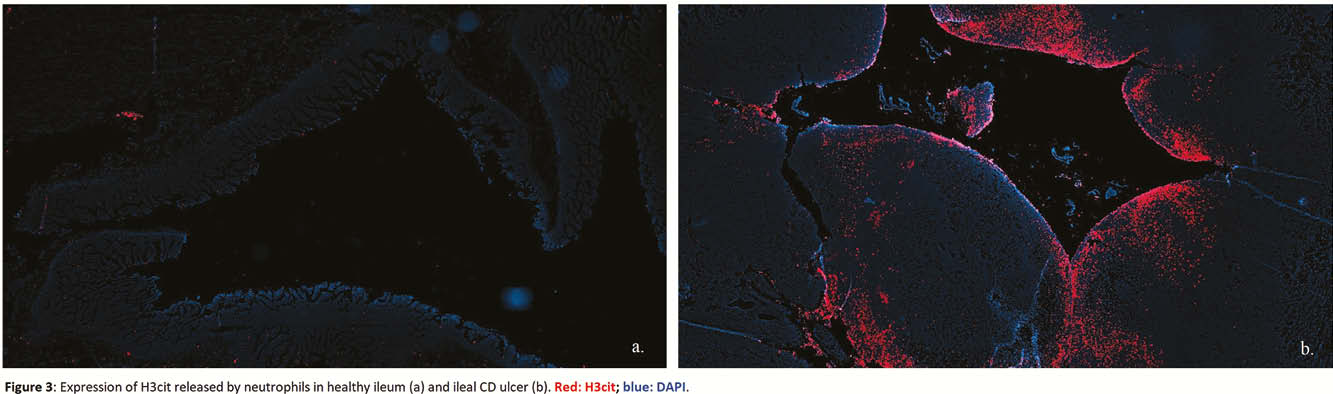
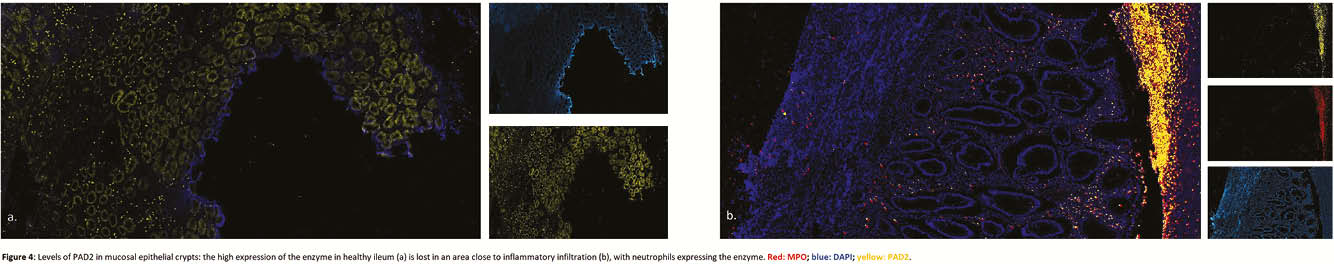
Conclusion
Both PAD2 and PAD4 are strongly expressed in neutrophils of CD ileal resection specimens, but only PAD4 shows a significantly higher expression in the inflammatory context which translates in the formation of NETs. No direct correlation was observed between PAD enzymes and intestinal fibroblasts.


