P068 Polyunsaturated fatty acid excess fuels pro-inflammatory transcriptional networks in GPX4 deficient intestinal epithelial cells
Meyer, M.(1)*;Grabherr, F.(1);Schwärzler, J.(1);Mayr, L.(1);Jukic, A.(1);Rao, Z.(2);Rieder, D.(3);Zollner, A.(1);Grander, C.(1);Trajanoski, Z.(3);Koeberle, A.(2);Tilg, H.(1);Adolph, T.E.(1);
(1)Medical University of Innsbruck, Department of Internal Medicine I- Gastroenterology- Hepatology- Endocrinology & Metabolism, Innsbruck, Austria;(2)University of Innsbruck, Michael Popp Research Institute, Innsbruck, Austria;(3)Medical University of Innsbruck, Biocenter- Institute of Bioinformatics, Innsbruck, Austria;
Background
There is increasing evidence that the rise in Crohn’s disease (CD) incidence is partially driven by constituents of a Western-style diet such as polyunsaturated fatty acids (PUFA). Recently we could demonstrate that PUFA excess induces a CD-like enteritis in mice lacking antioxidant GPX4 in their intestinal epithelial cells (IECs) and that CD patients clinical disease activity positively correlates with PUFA intake. In this project we investigated the role of epithelial lipid composition and lipid mediators during PUFA induced metabolic gut inflammation. Furthermore, we transcriptionally profiled GPX4 deficient small intestinal cells after stimulation with ω3- and ω6-PUFA.
Methods
Mice lacking one allele of Gpx4 in their IECs (GPX4+/-IEC) and wild-type controls (WT) were fed a PUFA (10% fish oil) enriched Western diet (PUFA-WD) for 12 weeks. Intestinal inflammation was assessed by histopathology. IECs were harvested by scraping of small intestinal sections and lipid profiles and mediators were analysed with LC/MS. GPX4 in murine small intestinal cells (MODE-K) was knocked down by siRNA (siGpx4) scrambled siRNA served as control (siCtrl). MODE-K were stimulated with ω3-, ω6-PUFA, 5,6- and 8,9-DHET or vehicle. Cytokine expression in cell culture supernatant was analysed by ELISA and transcriptome was analysed by bulk RNA sequencing.
Results
Histopathology revealed gut inflammation in GPX4+/-IEC but not WT mice after 12 weeks PUFA-WD feeding. LC/MS analysis of IECs derived from GPX4+/-IEC and WT mice fed the PUFA-WD depicted a significant increase in eicosapentaenoic acid (20:5), docosapentaenoic acid (22:5) and docosahexaenoic acid (22:6) containing phosphatidylethanolamine (PE) and phosphatidylcholine (PC) species (Fig 1a).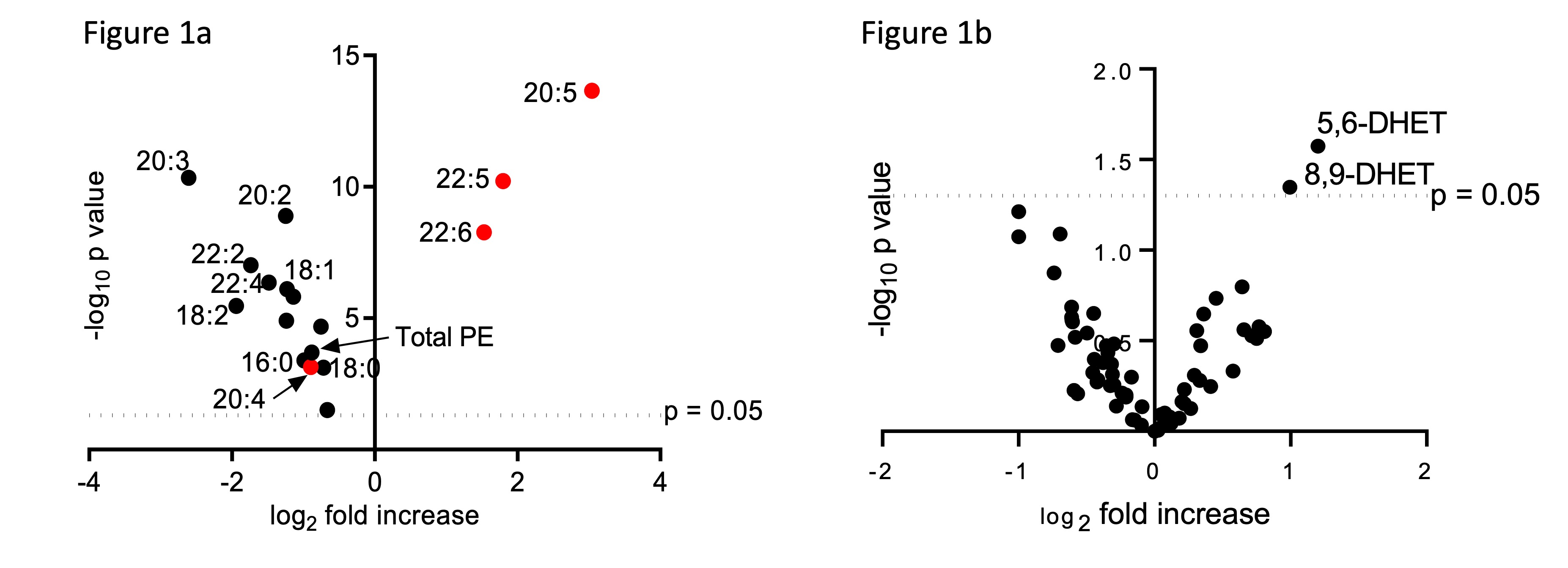
All other PE and PC species showed a reduced amount, confirming the enrichment of the specific PUFAs within the IECs by the PUFA-WD. Lipid mediator analysis further revealed an increase in 5,6-DHET and 8,9-DHET only in IECs derived from GPX4+/-IEC but not WT mice (Fig 1b). When stimulated with PUFA, siGpx4 MODE-K produced high amounts of neutrophil attracting chemokine CXCL-1 which is attenuated by the supplementation of anti-inflammatory lipid mediators 5,6-DHET and 8,9-DHET (Fig 2). Transcriptome analysis revealed the upregulation of pro-inflammatory pathways including TNFa, MAPK and NF-κB in siGpx4 vs. siCtrl MODE-K after PUFA stimulation (Fig 3). 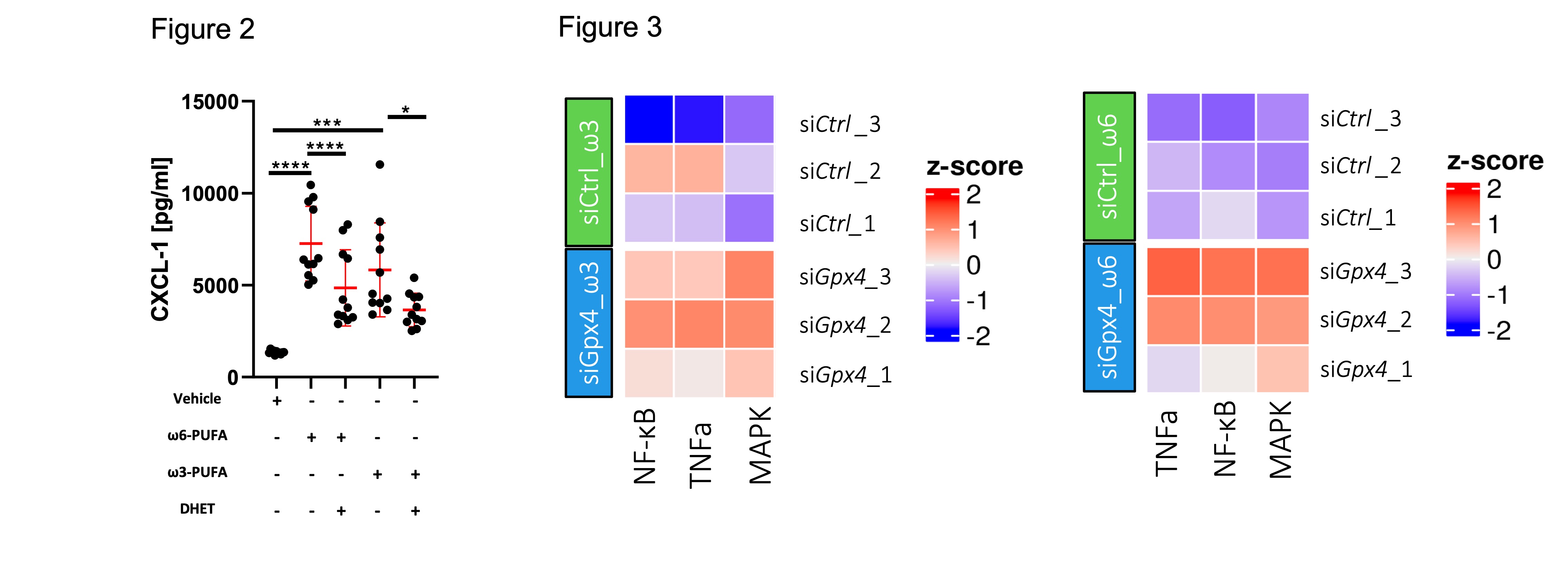
Conclusion
By integrating lipidomic and transcriptomic data of a reductionist cell culture system and a model organism, we could show that PUFA excess in a GPX4 deficient individual fuels a pro-inflammatory transcriptional network which outweighs anti-inflammatory ω3-PUFA metabolites influence culminating in gut inflammation.


