P079 High-dimensional mass cytometry after local MSC treatment in patients with refractory proctitis shows an effect on the myeloid compartment: preliminary data
L. Ouboter Drs.1,2, M.C. Barnhoorn1, L.J.A.C. Hawinkels1, M. van Pel2, J.J. Zwaginga2, H.W. Verspaget1, F. Koning2, M.F. Pascutti2, A.E. van der Meulen-de Jong1
1Leiden University Medical Center, Gastroenterology and Hepatology, Leiden, The Netherlands, 2Leiden University Medical Center, Immunohematology and Blood Transfusion, Leiden, The Netherlands
Background
Ulcerative proctitis (UP) causes a chronic rectal mucosal inflammation. A subset of patients does not respond to one or more medication options. Mesenchymal stromal cells (MSCs) show beneficial effects in animal models and patients with immune diseases. However, the effects of MSCs on the mucosal immune composition in patients with inflammatory bowel disease (IBD) are not unravelled yet.
Methods
Patients with UP (
Results
FCP decreased significantly in four out of seven patients (419→191; 365→40; 1744→346; 3270→291), ‘responders’, while in three patients FCP increased, ‘non-responders’. Unbiased hierarchical clustering of the subsets showed no clear differences in the major immune lineages before and after treatment. In some patients; however, a prominent myeloid population was present in the affected colon, whereas the CD8+− and CD3−CD7+ populations in affected tissue were in general less frequent compared with unaffected tissue from the same patients. We then zoomed into every major lineage separately, and observed changes in the frequencies of two myeloid clusters in the affected tissue of ‘responders’ compared with the ‘non-responders’ (see figure, Cluster 1=CD11b+CD11c+CD66b+CD15intCD16+CD45RA−CD45RO+ cells, Cluster 2= CD11b+CD11c+CD66b+ CD15−CD16−CD69+CD45RA+CD45RO+ cells).
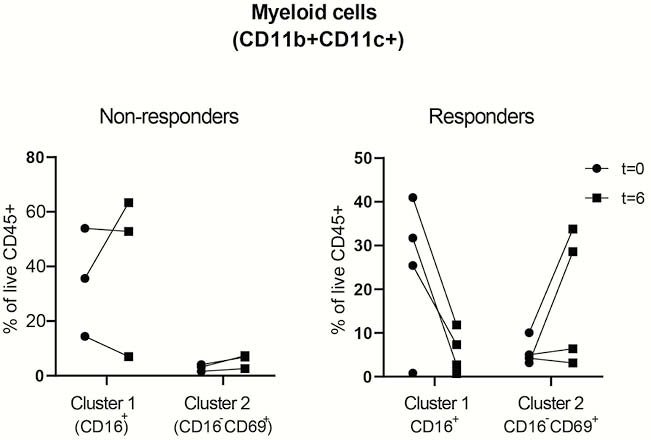
Conclusion
These preliminary data show differences between ‘responders’ and ‘non-responders’ in the myeloid compartment in the inflamed tissue after MSC therapy. Since CD11b+ and CD11c+ populations mediate the tolerogenic effect of MSC in mouse models, we will explore the regulatory potential of the observed populations in MSC-treated UP patients in future studies. The inclusion of patients in a second cohort (


