P102 Host gene background and concentration influence the butyrate effect on modulating IECs proliferation
H. He1, J. Hu2, W. Wang2, M. Zhang2, M. Zheng2, X. Peng2, Y. Zhang2, M. Zhou2, T. Liu2, J. Zhao2, Q. Zhang2, M. Zhi2,1
1The Second Affiliated Hospital of South China University of Technology, The Sixth Affiliated Hospital, Sun Yat-Sen University, Guangzhou First People’s Hospital, Guangdong Institute of Gastroenterology, Guangdong Province Key Laboratory of Colorectal and Pelvic Floor Diseases, Gastroenterology, Guangzhou, China, 2The Sixth Affiliated Hospital, Sun Yat-Sen University, Guangdong Institute of Gastroenterology- Guangdong Province Key Laboratory of Colorectal and Pelvic Floor Diseases, Gastroenterology, Guangzhou, China
Background
Intestinal epithelial barrier dysfunction is a major pathological feature of inflammatory bowel disease (IBD). Stability and accuracy of intestinal epithelial cells (IECs) self-renew are the fundament of intestinal mucosal regeneration and repair. Fermentable dietary fibre and short-chain fatty acid (SCFA) play a role in cell proliferation, histone acetylation and immune responses. However, the results about SCFA modulating IECs proliferation are not coincided. There are several reason that SCFA can have an impact on IECs through immune cell in animal experiment and that also include animal gene background, SCFA concentration, cell types used in cell experiment l. So we adopted intestinal organoids to investigate the effect of butyrate on IECs self-renew to avoid the immune cell influence and signal type cell experiments limitation.
Methods
Six to 8 weeks wide-type mice and IL10−/− mice were used. Mouse intestinal crypt isolation and organoid culture were according to previously published methods (H. Clevers et al.,
Results
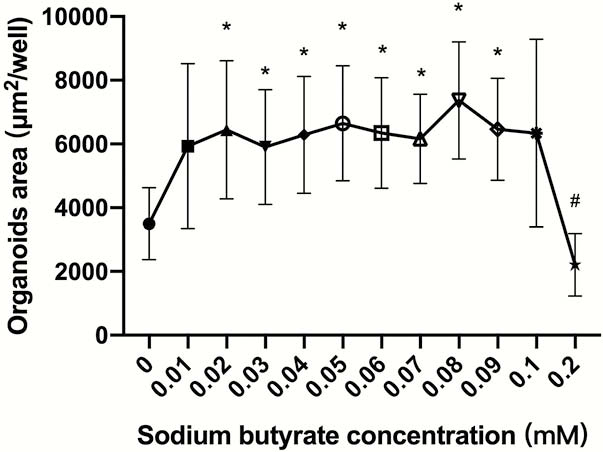
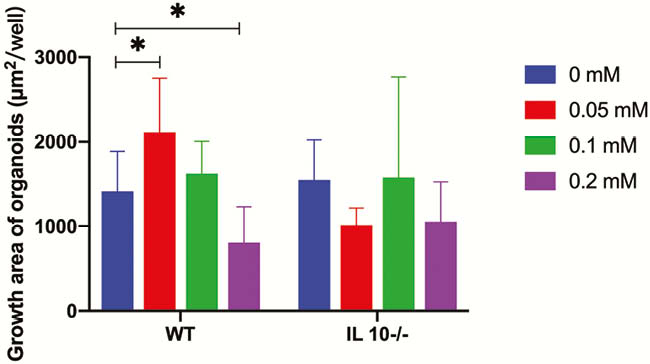
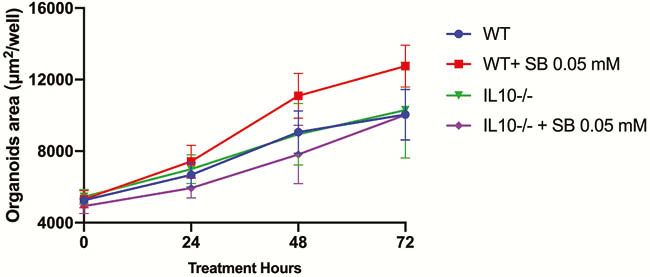
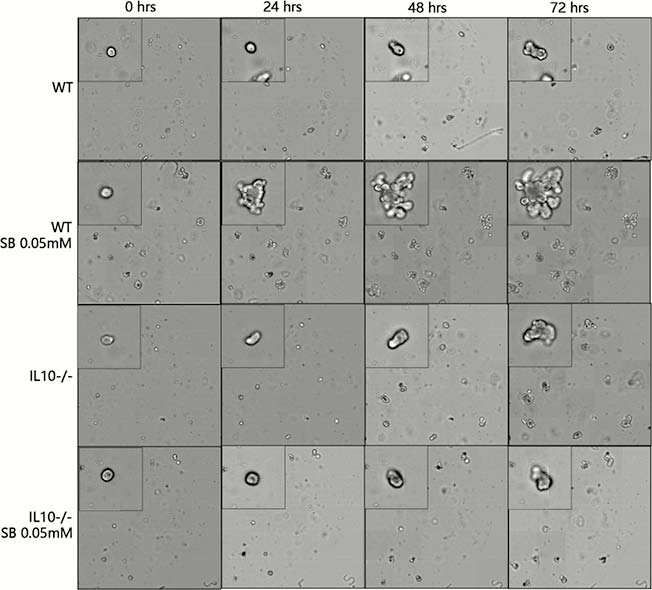
SB promoted intestinal organoids proliferation in a narrow effective concentration from 0.02 to 0.09 mM (Figure 1). The effective SB concentration applied to WT mice-derived organoids other than IL10−/− mice-derived organoids. SB can effectively and rapidly promote organoid proliferation (Figures 2 and 4). SB can continuously promote the proliferation of organoids-derived organoids (Figures 3 and 4).
Conclusion
There is a narrow effective SB concentrationpromotes WT mice-derived organoids other than IL10−/− mice-derived organoids. This result may be used to illustrate the phenomenon that IBD-associated microbiota and metabolites, butyrate, has different effects on IBD patient and IBD model mice.


