P136 Faecal calprotectin as therapeutic target in patients with Crohn’s disease treated with anti-TNF: a meta-analysis of individual data
A. Mami1, S. Nancey2, D. Pugliese3, P. Molander4, G. Boschetti2, I. Nogueira5, B. Pereira6, A. Buisson1
1CHU Estaing, Department of Digestive and Hepatobiliary Medicine- Department of Gastroenterology, Clermont-Ferrand, France, 2CHU Lyon-Sud, Gastroenterology, Lyon, France, 3Fondazione Policlinico A. Gemelli IRCCS- Rome- Italy, IBD Unit Presidio Columbus, Rome, Italy, 4Helsinki University Hospital- Meilahti Hospital, Hus, Helsinski, Finland, 5Federal University of São Paulo–UNIFESP-, Gastroenterology, Sao Paulo, Brazil, 6CHU, Biostatistics unit, Clermont-Ferrand, France
Background
Faecal calprotectin (Fcal) could be a non-invasive alternative to endoscopy to assess therapeutic efficacy in patients with Crohn’s disease (CD). We performed a systematic review and meta-analysis of individual data to assess the performances of FCal after anti-TNF induction therapy to predict mid-term steroids-free clinical remission (CFREM) in CD.
Methods
We included all the studies reporting patients with CD receiving anti-TNF agents with Fcal measurement before and after induction therapy (from 8 to 14 weeks) with follow-up > 6 months, no therapeutic intervention based on FCal during the follow-up and mid-term clinical evaluation between week 32 and week 54. CFREM was defined as CDAI < 150 and continuation of the same anti-TNF agent without steroids.
Results
We identified five articles including 165 patients with CD (mean age = 34.5 ± 12.8 years, median CD duration 5 years [2–12], female gende
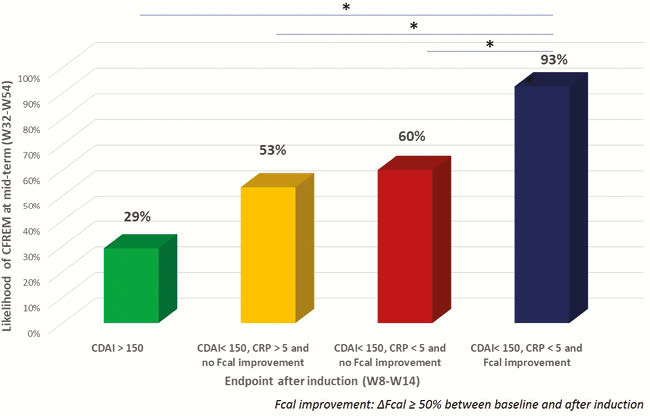
Conclusion
The improvement of Fcal after anti-TNF induction therapy is predictive of mid-term CFREM in patients with IBD. The combination of clinical remission, CRP normalisation and FCal improvement should be a therapeutic target in patients with CD. Our work highlighted the need of international standardisation of Fcal measurement.


